
The transition metals form a large number of complex compounds in which the metal atoms are bound to a number of anions or neutral molecules. In modern terminology such compounds are called coordination compounds.
Werner’s Theory of Coordination Compounds
The first systematic attempt at explaining the formation, reactions, structure and bonding of a coordination compound was made by A. Werner. The main postulates are:
- In coordination compounds metals show two types of linkages (valences)-primary and secondary.
- The primary valences are normally ionizable and are satisfied by negative ions.
- The secondary valences are non-ionizable. These are satisfied by neutral molecules or negative ions. The secondary valence is equal to the coordination number and is fixed for a metal.
- The ions/groups bound by the secondary linkages to the metal have characteristic spatial arrangements corresponding to different coordination numbers.
Difference between a double salt and a complex salt
- Both double salts, as well as complex salts, are formed by the combination of two or more stable compounds in a stoichiometric ratio.
- However, they differ in the fact that double salts such as carnallite, KCl.MgCl2.6H2O, Mohr’s salt, FeSO4.(NH4)2SO4.6H2O, potash alum, KAl(SO4)2.12H2O, etc. dissociate into simple ions completely when dissolved in water.
- However, complex ions such as [Fe(CN)6]4– of K4Fe(CN)6, do not dissociate into Fe2+ and CN– ions.
Coordination Compounds
A Co-ordination compound consists of a ligand, central atom, complex ion, a cation or an anion. The complex ion is generally written in a square box and the ion (cation or anion) is written outside complex ion.
eg: [Co (NH3)6]Cl3
[Complex ion] anion
eg: K4[Fe(CN)6]
cation [Complex ion]
General Formula: Ax[MLn]/[MLn]By
Where: M is the central metal atom/ion
L is the ligand
A is the cation
B is the anion
Some Important Terms pertaining to Coordination Compounds
Coordination entity:
It is the central metal atom or ion which is bonded to a definite number of ions or molecules which is fixed. For example, in [Co(NH3)6]Cl3, a coordination entity, six ammonia molecules are surrounded by three chloride ions.
Central atom/ion:
In a coordination entity, the atom/ion to which a fixed number of ions/groups are bound in a definite geometrical arrangement around it, is called the central atom or ion. For example, the central atom/ion in the coordination entities: [NiCl2(H2O)4], [CoCl(NH3)5]2+ and [Fe(CN)6]3– are Ni2+, Co3+ and Fe3+, respectively. These central atoms/ions are also referred to as Lewis acids.
Ligands:
The ions or molecules bound to the central atom/ion in the coordination entity are called ligands. These may be simple ions such as Cl–, small molecules such as H2O or NH3, larger molecules such as H2NCH2CH2NH2.
- When a ligand is bound to a metal ion through a single donor atom, as with Cl–, H2O or NH3, the ligand is said to be unidentate.
- When a ligand can bind through two donor atoms as in H2NCH2CH2NH2 (ethane-1,2-diamine) or C2O42- (oxalate), the ligand is said to be didentate.
- When several donor atoms are present in a single ligand as in N(CH2CH2NH2)3, the ligand is said to be polydentate.
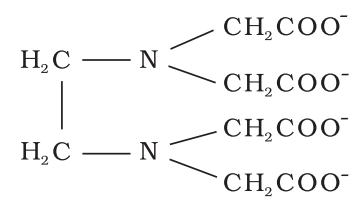
Co-ordination Number (C.N):
The number of atoms of the ligands that directly bound to the central metal atom or ion by co-ordinate bonds is known as the co-ordination number of the metal atom or ion. It is also equal to the secondary valency.
| Complex | Co-ordination numbers |
|---|---|
| K4[Fe(CN)6] | 6 |
| [Ag(CN)2]– | 2 |
| [Pt(NH3)2 Cl2] | 4 |
| [Ca(EDTA)]2- | 6 |
Coordination sphere:
The central atom/ion and the ligands attached to it are enclosed in square bracket and is collectively termed as the coordination sphere. The ionisable groups are written outside the bracket and are called counter ions. For example, in the complex K4[Fe(CN)6], the coordination sphere is [Fe(CN)6]4- and the counter ion is K+.
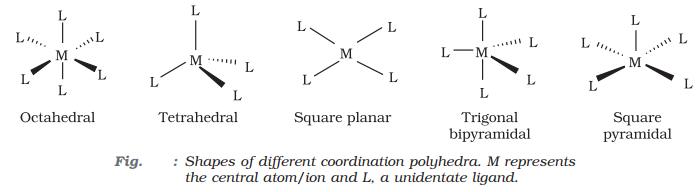
Coordination polyhedron:
A coordination polyhedron is the spatial arrangement of the ligand atoms that are directly attached to the central atom/ion. For example, [Co(NH3)6]3+ is octahedral, [Ni(CO)4] is tetrahedral and [PtCl4]2 is square planar.
Oxidation number of central atom:
It is defined as the charge that the central metal ion would carry if all the ligands are removed along with electron pairs.
It is calculate as follows:
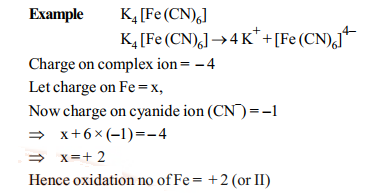
Homoleptic and Hetroleptic Complexes:
Complexes in which a metal is bound to only one kind of donor groups, e.g., [Co(NH3)6]3+, are known as homoleptic. Complexes in which a metal is bound to more than one kind of donor groups, e.g., [Co(NH3)4Cl2]+, are known as heteroleptic.

