
Methods of Preparation of Haloalkanes and Haloarenes
From Alcohols
The hydroxyl group of an alcohol is replaced by halogen on reaction with concentrated halogen acids, phosphorus halides or thionyl chloride to give the corresponding alkyl halide.

From Hydrocarbons
By free radical halogenation:
Free radical chlorination or bromination of alkanes gives a complex mixture of isomeric mono- and polyhaloalkanes, which is difficult to separate as pure compounds. Consequently, the yield of any one compound is low.
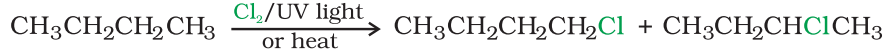
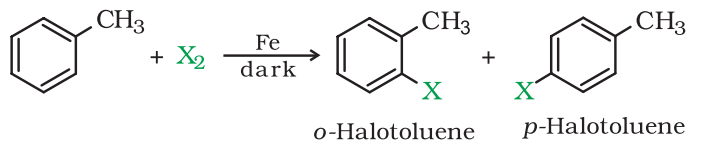
By electrophilic substitution:
Aryl chlorides and bromides can be easily prepared by electrophilic substitution of arenes with chlorine and bromine respectively in the presence of Lewis acid catalysts like iron or iron(III) chloride.
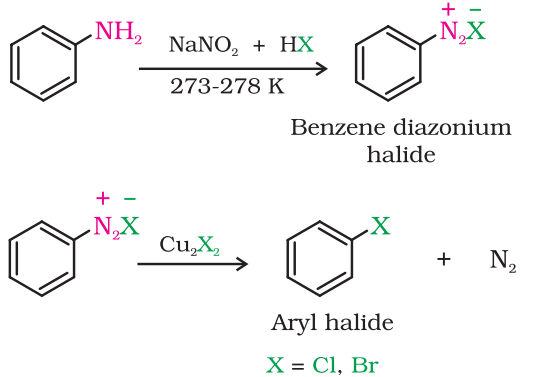
Sandmeyer’s reaction:
Aniline is treated with sodium nitrite to give a diazonium salt which is then treated with cuprous chloride or cuprous bromide to produce the corresponding aryl halide.
From alkenes:
Addition of hydrogen halides:
An alkene is converted to corresponding alkyl halide by reaction with hydrogen chloride, hydrogen bromide or hydrogen iodide.
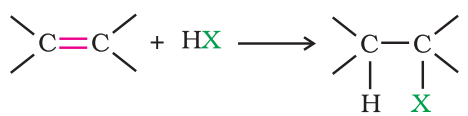
Propene yields two products, however only one predominates as per Markovnikov’s rule.
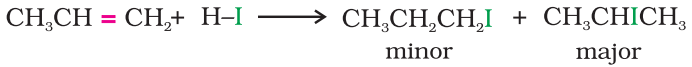
Addition of halogens:
In the laboratory, addition of bromine in CCl4 to an alkene resulting in discharge of reddish brown colour of bromine constitutes an important method for the detection of double bond in a molecule. The addition results in the synthesis of vic-dibromides, which are colourless.

Halogen Exchange
Finkelstein reaction:
Alkyl iodides can be prepared by the reaction of alkyl chlorides/ bromides with NaI in dry acetone.
![]()
Swarts reaction:
Alkyl fluorides can be prepared by heating an alkyl chloride/bromide in the presence of a metallic fluoride such as AgF, Hg2F2, CoF2 or SbF3.
![]()

