
Nitric acid
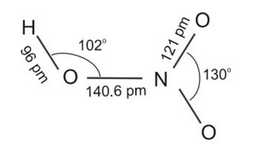
Nitric acid is the most important oxoacid formed by nitrogen. It is a colourless liquid. In the gaseous state, HNO3 exists as a planar molecule with the structure as shown below:
Preparation Nitric acid:
Nitric acid is manufactured by the catalytic oxidation of ammonia in Ostwald process.
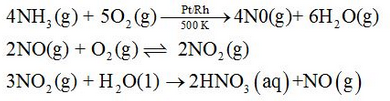
Laboratorically, nitric acid can be prepared by the heating NaNO3 or KNO3 with conc. H2SO4.
![]()
Properties of nitric acid:
Concentrated HNO3 is a strong oxidising agent and attacks most metals except noble metals. Cr, Fe and AI do not dissolve in conc. HNO3 due to the formation of a passive film of oxide on the surface.
The oxidising action of HNO3 is depends on its concentration and the nature of the reducing agent. The principal product of reduction of HNO3 is NO when it is dilute but NO2 when it is concentrated.
For example:
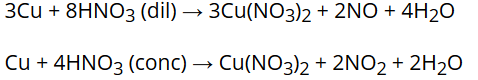
Brown ring test for the detection of nitrates:
Addition of dilute ferrous sulphate solution (Fe2+) to an aqueous solution containing nitrate ion, and then carefully adding concentrated sulphuric acid along the sides of the test tube results in the formation of a brown coloured complex [Fe(H2O)5 (NO)]SO4.
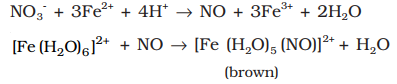
Uses of nitric acid:
- HNO3 is used in the manufacture of fertilisers.
- It is used in the formation of explosives, dynamites, TNT, etc.
- It is also used in the etching of metals.

