
Non-Stoichiometric Defects
A large number of non-stoichiometric inorganic solids are known which contain the constituent elements in non-stoichiometric ratio due to defects in their crystal structures.
These defects are of two types:
- Metal excess defect
- Metal deficiency defect.
Metal Excess Defect
Metal excess defect due to anionic vacancies:
Alkali halides like NaCl and KCl show this type of defect. When crystals of NaCl are heated in an atmosphere of sodium vapor, the sodium atoms are deposited on the surface of the crystal. The Cl– ions diffuse to the surface of the crystal and combine with Na atoms to give NaCl. This happens by loss of electron by sodium atoms to form Na+ ions. The released electrons diffuse into the crystal and occupy anionic sites (Fig.).
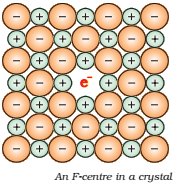
The anionic sites occupied by unpaired electrons are called F-centre. This F-centre is responsible for imparting color to the crystal. Like as:
NaCl – yellow color
LiCl – pink
KCl – violet
- It occurs in ionic compounds
- It is responsible for imparting color to the crystal through F-centres.
For example: NaCl, KCl, LiCl.
Metal excess defect due to the presence of extra cations at interstitial sites:
Zinc oxide is white in color at room temperature. On heating it loses oxygen and turns yellow.
![]()
Now there is excess of zinc in the crystal and its formula becomes Zn1+xO. The excess Zn2+ ions move to interstitial sites and the electrons to neighboring interstitial sites.
Metal Deficiency Defect
There are many solids which are difficult to prepare in the stoichiometric composition and contain less amount of the metal as compared to the stoichiometric proportion.
A typical example of this type is FeO which is mostly found with a composition of Fe0.95O. It may actually range from Fe0.93O to Fe0.96O.
In crystals of FeO some Fe2+ cations are missing and the loss of positive charge is made up by the presence of required number of Fe3+ ions

