Resonance Structure
A number of organic compounds cannot be accurately represented by one structure. For example, benzene is ordinarily represented as

This structure has three C-C bonds and three C=C bonds.
Carbon-carbon double bond length = 1.34 A
Carbon-carbon single bond length = 1.54A.
But it has been determined experimentally that all carbon-carbon bonds in benzene are identical and have same bond length (1.39A).
Thus, the structure of benzene cannot be represented by single structure. It can be represented equally well by ‘he energetically similar structures I and II. The two structures are called resonance structures.
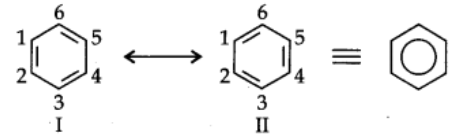
Actual structure of benzene is resonance hybrid of structures I and II.
Another example of resonance is provided by nitromethane (CH3N02) which can be represented by two Lewis structures.
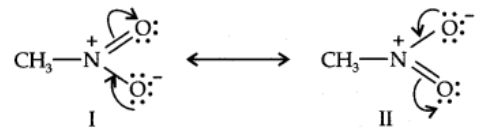
The actual structure of nitromethane is a resonance hybrid of the two canonical forms I and II.
Resonance energy:
The difference in the energy between the most stable contributing structure for a compound and its resonance hybrid is called as resonance energy or resonance stabilization energy.
Resonance Effect
The polarity produced in the molecule by the interaction of two π-bonds or between a π-bond and a lone pair of electrons present on an adjacent atom. There are two types of resonance or mesomeric effects designated as R or M effect.
Positive Resonance Effect (+R effect)
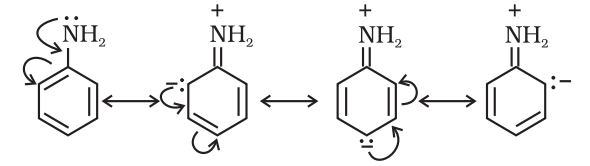
Those atoms which lose electrons towards a carbon atom are said to have a +M effect or +R effect. For example:
-Cl, -Br, -I, -NH2, -NR2, -OH, -OCH3
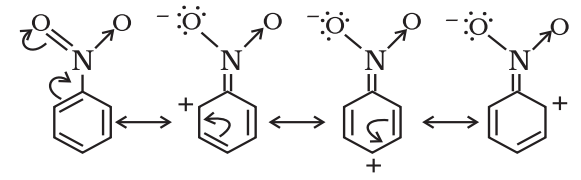
Negative Resonance Effect (-R effect)
Those atoms or groups which draw electrons away from a carbon atom are said to have a -M effect or -R effect.
For example: -NO2, -C≡N, -CHO