
Chemical reactions involve the breaking and making of bonds between atoms to produce new substances. On basis of bond formations chemical reactions can be classified into following types.
1. Combination Reaction:
CaO + H2O → CaOH2
In this reaction, calcium oxide and water combine to form a single product, calcium hydroxide. Such a reaction in which a single product is formed from two or more reactants is known as a combination reaction.
2. Decomposition Reaction :
2FeSO4 → Fe2O3 + SO2 + SO3
In this reaction you can observe that a single reactant breaks down to give simpler products. This is a decomposition reaction.
3. Displacement Reaction:
Fe + CuSO4 → FeSO4 + Cu
In this reaction, iron has displaced or removed another element, copper, from copper sulphate solution. This reaction is known as displacement reaction.
4. Double Displacement Reaction:
Na2SO4 + BaCl2 → BaSO4 + 2NaCl
Such reactions in which there is an exchange of ions between the reactants are called double displacement reactions.
5. Oxidation and Reduction:
2Cu + O2 → 2CuO
If hydrogen gas is passed over this heated material (CuO), the black coating on the surface turns brown as the reverse reaction takes place and copper is obtained.
CuO +H2 → Cu+H2O
If a substance gains oxygen during a reaction, it is said to be oxidised. If a substance loses oxygen during a reaction, it is said to be reduced.
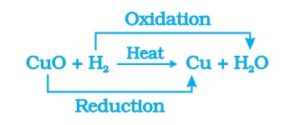
During this reaction , the copper oxide is losing oxygen and is being reduced. The hydrogen is gaining oxygen and is being oxidised. In other words, one reactant gets oxidised while the other gets reduced during a reaction. Such reactions are called oxidation-reduction reactions or redox reactions.

