
Ammonia
Ammonia molecule is trigonal pyramidal with the nitrogen atom at the apex. It has 3 bond pairs and 1 lone pair. N is sp3 hybridized.
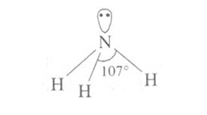
Ammonia is highly soluble in water and is weakly basic.
![]()
Preparation of Ammonia:
Ammonia (NH3) is manufactured on the commercial scale by Haber’s process.
![]()
- Pressure ‒ 200 × 105 Pa
- Temperature ‒ 773 K
- Catalyst ‒ Iron oxide with small amounts of K2O and Al2O3. In the laboratory, ammonia is prepared by reacting NH4Cl with NaOH.
![]()
Properties of Ammonia:
Due to the presence of the lone pair of electrons on the nitrogen atoms, NH3 is a Lewis base. It can form a coordinate covalent bond with the transition metal ion and form complexes, e.g.
![]()
Aqueous NH3 is also used in the precipitation of metal ions, e.g.
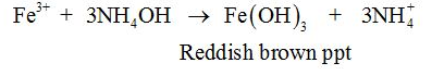
Uses of Ammonia:
- It is used as a refrigerant.
- It is used in the manufacture of nitric acid.
- It is used in the production of nitrogenous fertilizers.

