
Nature of C-X Bond
X is more electronegative than carbon. So, the C-X bond is polarized with C having a partial positive charge and X having a partial negative charge.
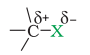
Since the size of halogen atom increases as we go down the group in the periodic table, fluorine atom is the smallest and iodine atom, the largest.


