
Enthalpy of fusion:
Enthalpy of fusion is the heat energy or change in enthalpy when one mole of a solid at its melting point is converted into liquid state.
For example: Enthalpy of fusion of ice at 273 K is 6.0 kJ mol-1
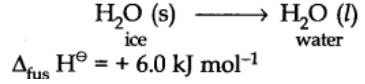
Enthalpy of vaporization:
It is defined as the heat energy or change in enthalpy when one mole of a liquid at its boiling point changes to gaseous state.
For example:
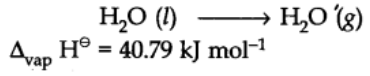
Enthalpy of Sublimation:
Enthalpy of sublimation is defined as the change in heat energy or change in enthalpy when one mole of solid directly changes into gaseous state at a temperature below its melting point.
For example:
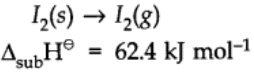
Standard Enthalpy of Formation
Enthalpy of formation is defined as the change in enthalpy in the formation of 1 mole of a substance from its constituting elements under standard conditions of temperature at 298K and 1 atm pressure.
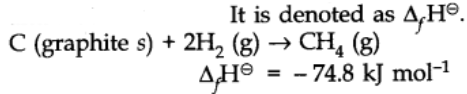
Enthalpy of combustion:
It is defined as the heat energy or change in enthalpy that accompanies the combustion of 1 mole of a substance more than air or oxygen.
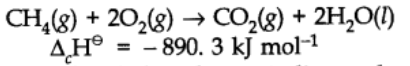
The negative sign of enthalpy change indicated that this is an exothermic reaction.

