
Human body has about 65% and some plants have nearly 95% water.
Water is the most abundant and widely distributed on the earth. it occurs in all the three physical states. H2o is a covalent molecule in which oxygen is sp3 hybridised. It has bent structure.

Physical properties of water:
- Freezing point of water is 273.15 K and boiling point 373.15 K.
- Maximum density of water at 4oC is 1 gm cm-3.
- It is a colourless and tasteless liquid.
- Due to hydrogen bonding with polar molecules, even covalent compounds like alcohol and carbohydrates dissolve in water.
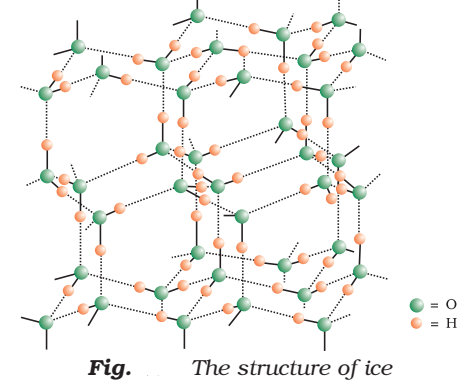
Structure of Ice
Chemical Properties of Water
Amphoteric nature:
It behaves like an amphoteric substance because it can act as an acid as well as base.
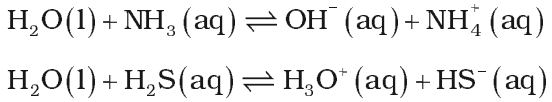
Autoprotolysis of water also accounts for its amphoteric nature according to Bronsted-Lowry concept.

pH of water is 7. And it is neutral towards pH.
Oxidising and Reducing Nature:
Water can act as an oxidising as well as reducing agent.
As an oxidising agent:
![]()
As reducing agent:
![]()
Hydrolysis Reaction:
It has a very strong hydrating tendency. It can hydrolyse a large number of compounds such as oxides, halides, carbides etc.
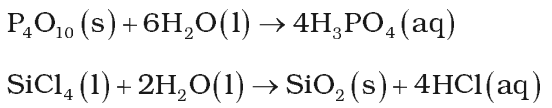
Hydrates Formation:
From aqueous solutions many salts can be crystallised as hydrated salts. Hydrates are of three types:
Coordinated water:
For example:
![]()
- Interstitial water
For example: BaCl2. 2H2O
- Hydrogen bonded water
For example:
![]()
Hard and Soft Water
Hard water
Water which does not produce lather with soap easily is called hard water. Presence of calcium and magnesium salts in the form of hydrogen carbonate, chloride and sulphate in water makes the water hard.
Types of Hardness of Water
Temporary Hardness:
It is due to the presence of bicarbonates of calcium and magnesium in water.
Removal of temporary Hardness
It can be achieved:
- By boiling: The soluble bicarbonates are converted into insoluble carbonates.
- By Clark’s process: By adding lime water or milk of lime.
Permanent Hardness:
It is due to the presence of chlorides and sulphates of calcium and magnesium.
Removal of Permanent Hardness
- By adding washing soda: The calcium or magnesium salts are precipitated as carbonates.
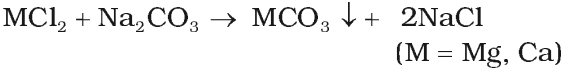
- By adding caustic soda: The temporary and permanent hardness can be removed by adding caustic soda.
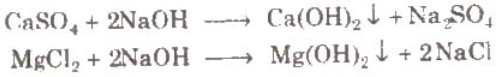
- Calgon’s process: Calgon is sodium hexa metaphosphate (Na6P6O18). This calgone when added to hard water form soluble complex.
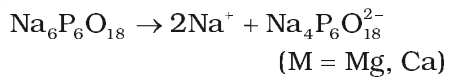
Similarly, Mg2+ can also precipitate as Na2[Mg2(PO3)6] and water becomes free from Ca2+ and Mg2+ Ions.
- Permutit process: Permutit is hydrated sodium aluminium silicate Na2Al2Si2O8.xH2O. It exchanges its sodium ions for divalent ions Ca2+ and Mg2+.
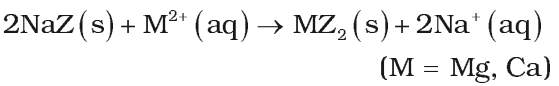
Permutit when fully exhausted can be regenerated by treating with 10% solution of sodium chloride. It is most efficient method to gel water with zero degree of hardness.
- By synthetic resins:
- Cation exchange resins
- Anion exchange resins
Soft Water
Water which readily forms lather with soap is called soft water.
For example: Rainwater, distilled water

