
Actinoids
The 14 elements immediately following actinium (89), with atomic numbers 90 (Thorium) to 103 (Lawrencium) are called actinoids. They belong to second inner transition series. In actinoids the filling of electrons takes place in the anti-penultimate subshell.
General properties of actinoids:
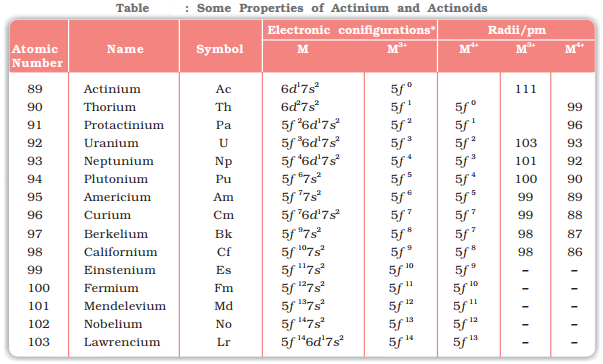
- Electronic configuration: The general electronic configurations for the actinoids is [Rn]5f1−146d0−17s2, where Rn is the electronic configuration of the element Radium. The fourteen electrons are formally added to though not in Thorium but onwards from it and the 5f subshell is complete at Lr (Z = 103). The irregularities in the electronic configuration of the actinoids are related to the stabilities of empty, half-filled or filled f subshell.
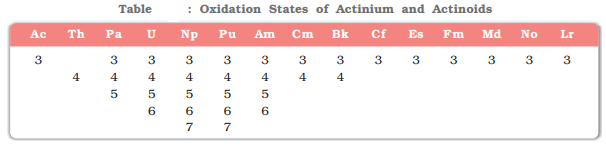
- Oxidation state: The dominant oxidation state of actinoids is +3. However, they also show variable oxidation states due to the comparable energy of 5f, 6d and 7s subshells. For example, The uranium shows oxidation states of and. The element neptunium (Z = 93) shows an oxidation state up to +7.
- Magnetic character: Actinoids also show paramagnetic but their magnetic properties are much more complex than those of the lanthanoids.
- Atomic and ionic sizes: In actinides, the ionic radii decrease as we move down the series. This decrease in ionic radius is termed as actinide contraction. This effect is due to poor screening offered by 5f electrons.
- Ionization enthalpy: The ionization enthalpies of actinoids are lower than those of corresponding lanthanoids. This is because the orbitals in actinoids penetrate less into the inner core of electrons, and the electrons are more effectively shielded from the nuclear charge than are the electrons of the corresponding lanthanoids. As the outer electrons are less tightly held, less amount of energy is required to ionize an atom.
- Metallic character: The actinoids are all metals with the silvery appearance. These metals are highly reactive when finely divided.
Chemical properties of Actinoids:
- They react with boiling water to give a mixture of oxide and hydride.
- All these metals are attacked by HCl but slightly affected by HNO3 due to the formation of a protective oxide layer on their surface.
- They combine with most of the non-metals at moderate temperature.

