
Inner Transition elements (f- Block) Introduction
The elements in which the differentiating electron enters the penultimate energy level i.e. (n−2)f, are called f-block elements. Due to such electronic configuration where the last electron enters the 4f or 5f orbitals that are lower than the outermost electrons, f-block elements are also named as inner transition elements.
Depending upon the fact whether the last electron enters the 4f or 5f-orbitals, f-block elements are differentiated into lanthanoids and actinoids.
Lanthanoids:
The 14 elements immediately following lanthanum, i.e., Cerium (58) to Lutetium (71) are called lanthanoids. They belong to first inner transition series. Lanthanum (57) has similar properties.
General properties of lanthanoids:
- Electronic configuration of lanthanoids: The general electronic configuration of the lanthanoids is
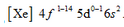

- Atomic and ionic Sizes: The decrease in atomic and ionic radii from lanthanum to lutetium is not quite regular but there is a regularity in the size of M3+ ions. The regular decrease in size of M3+ ion is attributed to the imperfect shielding of one electron by another in the same 4f subshell. This regular decrease in size amongst lanthanides as atomic number increases is known as the lanthanoid contraction.
- Formation of coloured ions: Lanthanide form ions which are coloured in both solid state and in aqueous solutions. Colour of these ions may be attributed to the presence of f-electron.
Exception: Lu3+ ions do not show any colour due to the absence of any unpaired electron in the 4f subshell which is fully filled.
- Magnetic character: Lanthanide ions also show paramagnetism. In lanthanides, the magnetic moment is due to both spin magnetic moment as well as orbital magnetic moment.
- Oxidation state: The most common oxidation state of lanthanides is + 3 which is obtained by using two electrons in 6s and one electron from 5d subshell.
Exception: Some elements show +2 and +4 oxidation states. This irregularity arises mainly from the extra stability of empty, half-filled or filled f subshell.
For example:

Physical properties of lanthanoids:
- All the lanthanoids are silvery white soft metals and tarnish rapidly in air, the hardness increases with increasing atomic number.
- The melting points range between 1000 to 1200 K but samarium melts at 1623 K.
- They are also good conductors of heat and electricity.
Chemical properties of lanthanoids:
- Some important chemical reactions of lanthanoids are:
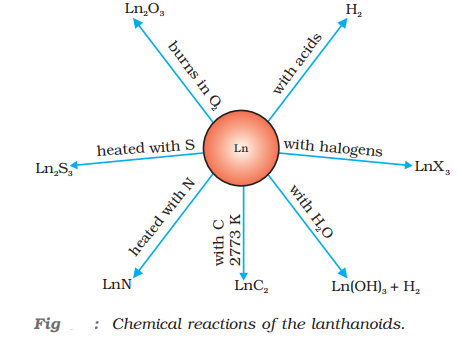
Formation of alloys:
Lanthanoids are all used in the steel industry for making alloy steels. The important and well-known alloy is misch-metal and it consists of lanthanoid (90-95%), iron (4-5%) and the trace amount of S, C Ca and Al.
Uses of lanthanoids
- Misch-metal is used in making tracer bullets, shell and lighter flint.
- Mixed oxides of lanthanoids are used as a catalyst in petroleum cracking. Some individual oxides of lanthanoids are used as phosphors in television screens and similar fluorescing surface.

