
Alcohols
The compounds in which hydroxyl group (-OH) is attached to a saturated carbon atom are called as Alcohols.
Classification of alcohols
#1 On the basis of the number of carbons attached to the α- carbon atom, i.e., the carbon to which the OH group is attached, alcohols are classified as:
- Primary (10) alcohol: This type of alcohol has only one carbon atom attached to the α- carbon atom. For example:
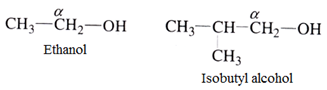
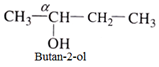
- Secondary (20) alcohol: Here α- carbon has two other carbon atoms attached to it. For example:
- Tertiary (30) alcohol: In such alcohol three other carbon atoms are attached to the α- carbon atom.
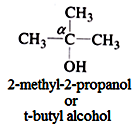
#2 On the basis of the number of hydroxyl groups (-OH) present, alcohols can be divided into the following categories:
- Monohydric alcohols: They contain only one OH group. They have a general formula CnH2n+2O. For example: CH3OH, CH3CH2OH, etc.
- Dihydric alcohols: Such alcohol two OH groups. For example:
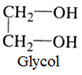
- Trihydric alcohols: This type of alcohols contains three OH groups. For example:
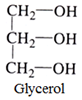
- Polyhydric alcohols: These are the alcohols that contain more than three OH groups.

