
Anomalous Properties of Boron
Boron shows anomalous behavior with the other members of the group, due to the following reasons:
- Smallest size in the group
- High ionization energy
- Highest electronegativity in the group.
- Absence of vacant d-orbital
Some Important Compounds of Boron (B)
Borax
- Borax is a white crystalline solid.
- It contains tetranuclear units. [B4O5(OH)4]2-
- It formula is
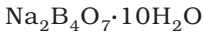
- Borax dissolves in water to give an alkaline solution
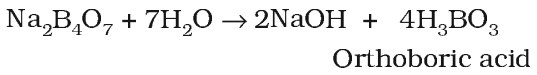
- Borax is used as a water softener and cleaning agent.
Orthoboric acid (H3BO3) or B(OH)3
Boric acid can be prepared by the acidification of aqueous solution of borax.
![]()
It can also be prepared by the hydrolysis of boron compounds.
![]()
Physical properties of Boric acid
- It is a white crystalline solid.
- It is soft soapy in touch.
- It is sparingly soluble in cold water but fairly soluble in hot water.
Uses of Boric acid
- In the manufacture of heat resistant borosilicate glazes.
- As a preservative for milk and food stuffs.
- In the manufacture of enamels and glazes in pottery.
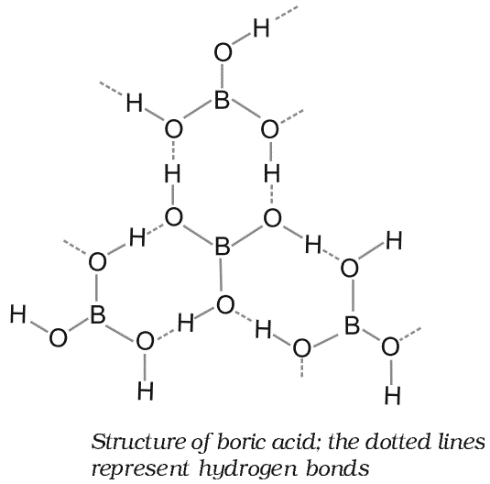
Structure of Boric Acid
Diborane (B2H6)
- The series of compounds of boron with hydrogen is known as boranes.
- Diborane is prepared by the reduction of boron trifluoride with LiAlH4 in diethyl ether.
![]()
- Laboratory method of preparation. In laboratory diborane is prepared by the oxidation of sodium borohydride with iodine.
![]()
- Industrial method of preparation. On industrial scale, diborane is prepared by reduction of BF3 with sodium hydride.
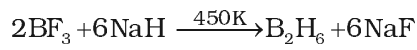
Structure of Diborane
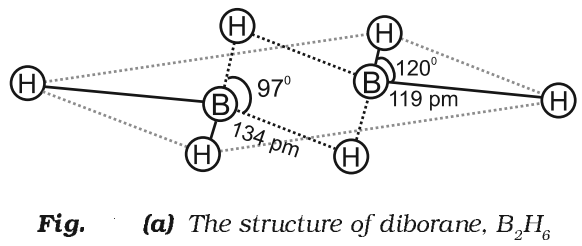
Physical Properties of Diborane
- Diborane is a colourless, highly toxic gas with a b.p. of 180 K.
- Diborane catches fire spontaneously upon exposure to air.
- Higher boranes are spontaneously flammable in air.
Chemical Properties
- Boranes are readily hydrolyzed by water to form boric acid.
![]()
- It burns in oxygen evolving an enormous amount of heat.
![]()
- Reaction with Lewis base: Diborane on treatment with lewis bases undergo cleavage reactions to form borane which then reacts with Lewis bases to form adducts.


