
Chemical Reactivity and E0 Values:
The transition metals vary very widely in their chemical reactivity. Some of them are highly electropositive and dissolve in mineral acids whereas a few of them are ‘noble’, i.e., they do not react with simple acids. Some results of chemical reactivity of transition metals as related to their E0 values are given below:
- The metals of the first transition series (except copper) are relatively more reactive than the other series. Thus, they are oxidized by H+ ions though the actual rate is slow, e.g., Ti and V are passive to dilute non-oxidizing acids at room temperature.
- As already explained, less negative E0 values for M2+/M along the series indicate a decreasing tendency to form divalent cations.
- More negative E0 values than expected for Mn, Ni and Zn show greater stability for Mn2+, Ni2+ and Zn2+.
- E0 values for the redox couple M3+/M2+ indicate that Mn3+ and Co3+ ions are the strongest oxidizing agents in aqueous solution whereas Ti2+, V2+ and Cr2+ are strongest reducing agents and can liberate hydrogen from a dilute acid, e.g.,
![]()
Magnetic Properties of d-block elements:
When a magnetic field is applied to substances, mainly two types of magnetic behaviour are observed: diamagnetism and paramagnetism (Unit 1). Diamagnetic substances are repelled by the applied field while the paramagnetic substances are attracted.
Most of the transition metals are paramagnetic in nature due to the presence of unpaired electrons. It increases from Sc to Mn due to the increased number of unpaired electrons and then starts decreasing as the number of unpaired electrons decreases.
In first transition elements series, the orbital angular magnetic moment is insignificant the orbital contribution is quenched by the electric fields of the surrounding atoms so magnetic moment is equal to the spin magnetic moment only
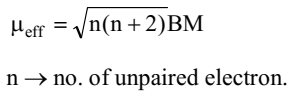
Maximum transition elements and compounds are paramagnetic due to presence of unpaired electrons.

