
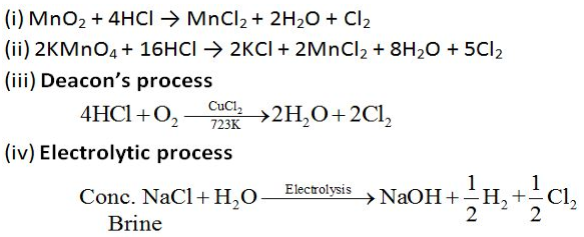
Preparation of Chlorine:
Chlorine can be prepared by any of the following processes:
Properties of Chlorine:
- It is a greenish yellow gas with pungent and suffocating odour.
- It is soluble in H2O
Reaction with metals and non-metals:
Chlorine reacts with a number of metals and non-metals to form chlorides. For example:
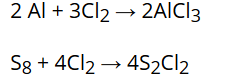
Reaction with ammonia:
When treated with excess ammonia, chlorine gives nitrogen and ammonium chloride whereas when excess chlorine reacts with ammonia, nitrogen trichloride is formed.

Reaction with NaOH:
Chlorine reacts differently with cold dilute NaOH and hot concentrated NaOH.
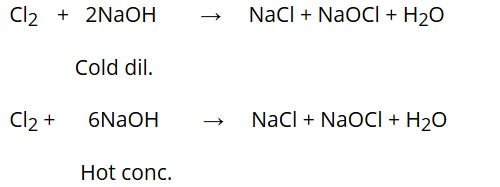
Reaction with slaked lime:
Cl2 when treated with dry slaked lime it gives bleaching powder:
![]()
Cl2 acts as a powerful bleaching agent and its bleaching action are due to its oxidizing nature.
![]()
Uses of Chlorine:
- Chlorine is used for bleaching wood pulp.
- It is used in the extraction of gold and platinum.
- It is used in in sterilising drinking water.
- It is used in the manufacture of dyes, drugs and organic compounds like CCl4, DDT, refrigerants etc.

