“The elements in which the last differentiating electron enters into the d-orbitals of the penultimate shell i.e. (n–1) d where n is the last shell is called d-block elements”. A transition element may also be defined as the element which partially filled d-orbital in their ground state or most stable oxidation state.
The properties of these elements are intermediate between the properties of s-block and p-block elements. These elements represent a change or transition in properties from more electropositive elements (s-block) to less electropositive elements (p-block). Therefore, these elements are called transition elements.
Position in the periodic table:
The position of the d-block element has been shown in periodic table as follows:
- d-block elements lie in between ‘s’ and ‘p’ block elements, i.e. these elements are located in the middle of the periodic table.
- d-block elements are present in:
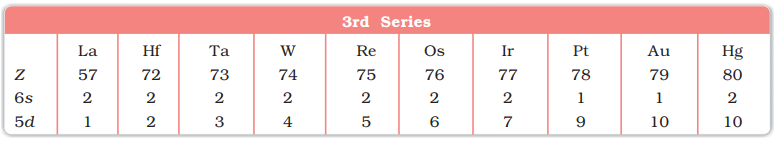
- 4th period (21Sc to 30Zn, 10 elements) 1st Transition series.
- 5th period (39Y to 48Cd, 10 elements) 2nd Transition series.
- 6th period (51La, 72Hf to 80Hg, 10 elements) 3rd Transition series.
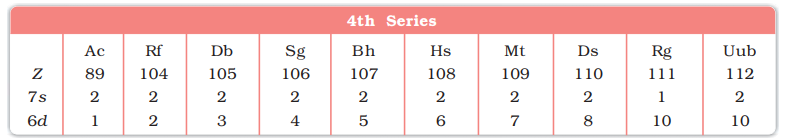
- 7th period (89Ac, 104Rf to 112Uub, 10 elements) 4th Transition series
Thus, the electronic configuration of chromium (Z = 24) and copper (Z = 29) are 1s2 2s2 2p6 3s2 3p6 3d5 4s1 and 1s2 2s2 2p6 3s6 3p6 3d10 4s1 respectively.
Electronic Configuration of the d-Block Elements:
- The general electronic configuration of d-block elements is (n −1)d1─10 ns1─2, where (n −1) stands for the inner d orbitals.
- In d-block, each horizontal row consists of ten elements as d-subshell can accommodate a maximum of 10 electrons.
- Depending upon the d-orbitals of which penultimate shell i.e. n = 4, 5, 6, 7 are filled, four rows (called series) or ten elements each obtained. They correspond to 3d, 4d, 5d and 6d subshells
- Energy of ‘(n–1)d’ subshell is slightly greater than ‘ns’ subshell, hence orbital is filled first then (n – 1) d orbitals.
- The general electronic configuration of d-block/d-series elements be shown as follows:
First (3d) Transition Series (Sc-Zn)
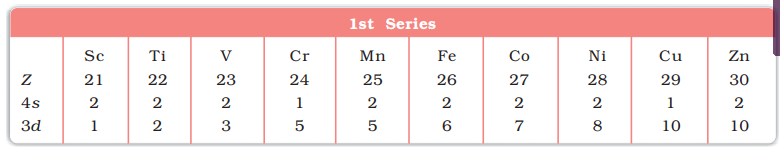
First (4d) Transition Series (Y-Cd)
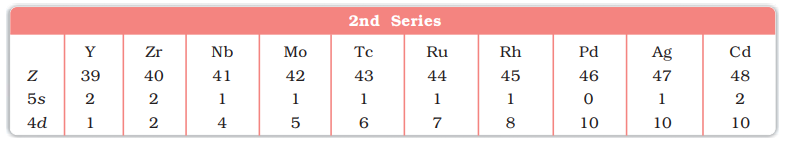
First (5d) Transition Series (La-Hg)
First (6d) Transition Series