Properties of d-Block elements:
Atomic Radii of d-Block elements:
- The atomic radii of the transition metals lie in-between those of s- and p-block elements.
- Generally, the atomic radii of d-block elements in a series decrease with increase in atomic number but the decrease in atomic size is small after midway.
- At the end of the period, there is a slight increase in the atomic radii.
- The atomic radii increase down the group. This means that the atomic radii of second series are larger than those of first transition series. But the atomic radii of the second and third transition series are almost the same.
- The atomic radii of the elements of the second and third transition metals are nearly same due to lanthanide contraction (or also called lanthanoid contraction).

Ionic radii of d-Block elements:
- The trend followed by the ionic radii is the same as that followed by atomic radii.
- Ionic radii of transition metals are different in different oxidation states.
- The ionic radii of the transition metals are smaller than those of the representative elements belonging to the same period.
Metallic Character of d-Block elements:
- Almost all the transition elements display metallic properties such as metallic luster, high tensile strength, ductility, malleability and high thermal and electrical conductivity.
- In any row, the melting point of these metals rises to a maximum at d5 and after that as the electrons start pairing up so the melting point decreases regularly as the atomic number increases with an exception of Mn and Tc are exception.
Melting Point of d-Block elements:
- Due to the strong interatomic bonding which involves both (n−1)d and ns electrons participation, transition metals have high melting points.
Ionisation energies or Ionisation enthalpies of d-Block elements:
- In a particular transition series, there is an increase in ionization enthalpy from left to right which is due to the increase in effective nuclear charge along a series. But the trend is not very regular.
- The exceptions are chromium and copper which have notably larger ionization enthalpy than their neighbours.
- These exceptions are due to the extra stability associated with the half-filled and fully-filled set of d-orbitals.
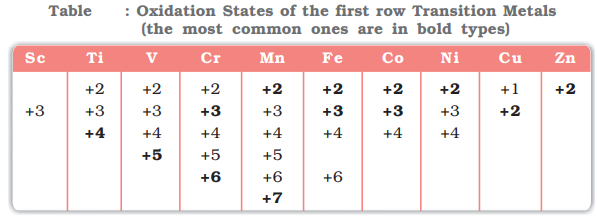
Oxidation State of d-Block elements:
- “The net numerical charge assigned to an atom of an element in its combined state is known as its Oxidation state or Oxidation number”
- Minimum oxidation state = Total number of electrons in 4s lost.
- Maximum oxidation state = (Total number of electrons in 4s + number of unpaired electrons in 3d lost)
- Transition metals show variable oxidation states due to tendency of (n-1)d as well as ns electrons to take part in bond formation.