
Preparation of Dinitrogen
- By heating a mixture of NH4Cl and NaNO2. N2 is collected by the downward displacement of water.
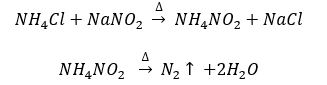
By treating an aqueous solution of ammonium chloride with sodium nitrate. It is laboratory method of preparation
![]()
- By heating ammonium dichromate:
![]()
- By oxidation of ammonia.
Properties of Dinitrogen
- N2 is a colourless, odourless gas insoluble in water
- It is non-polar covalent and neutral molecule.
- It is neither combustible nor a supporter of combustion.
- It is absorbed by heated Mg and Al. The nitrides formed thus react with water to form NH3.
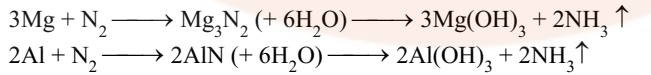
- Reaction with H2: At 200 atm and 500oC, and in the presence of iron catalyst and molybdenum promoter, N2 combines with H2 reversibly to form ammonia. The process is called Haber’s Process and is the industrial method of manufacturing ammonia. The reaction is exothermic.
![]()
- Reaction with oxygen: When air free from CO2 and moisture is passed over an electric arc at about 2000 K,
nitric oxide is formed. This reaction is endothermic
![]()
- Reaction with CaC2 and BaC2: At 1100oC, these carbides react with N2 forming CaCN2 and Ba(CN)2 respectively.
![]()
CaCN2 reacts with H2O in the soil to produce NH3 gas. NH3 gas is converted by the nitrating bacteria present in soil into nitrates. (The nitrates are readily absorbed by the plants and meet their requirement of the element nitrogen.)
Uses of Dinitrogen:
- for providing an inert atmosphere during many industrial processes where presence of air or O2 is to be avoided.
- for the manufacture of NH3 by the Haber’s process.
- for the manufacture of HNO3 by the Birkeland-Eyde process.
- for the manufacture of nitrolim.

