
Extraction of iron from its oxides
Oxide ores of iron, after concentration through calcination/roasting (to remove water, to decompose carbonates and to oxidise sulphides) are mixed with limestone and coke and fed into a Blast furnace from its top. Here, the oxide is reduced to the metal. Thermodynamics helps us to understand how coke reduces the oxide and why this furnace is chosen.

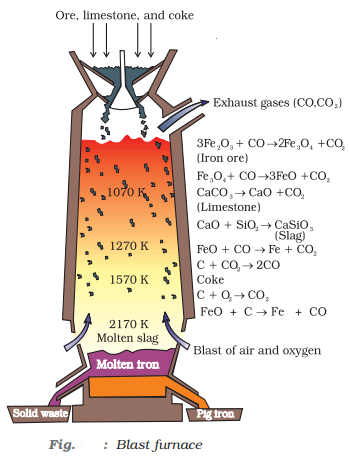
When the molten pig iron is cooled at once, the iron is called white cast iron, which contains carbon in the form of cementite, Fe3C and when the molten pig iron is cooled slowly and slowly, the iron is called as grey cast iron, which contains carbon in the form of graphite:
- Cost Iron or Pig Iron: This is the least pure form of commercial iron and contains the highest percentage of carbon viz., 2.5 to 4.5% and traces of impurities like S, P, Mn and Si. The average composition of cast iron is: Fe = 93 – 95%, C = 2.5 – 4.5%, Si = 0.6 – 2.8%, P = 0.4 – 1.0%, S = 0.1 – 0.3%, Mn = 0.3 – 1.2%.
- Wrought Iron: It is the purest form of commercial iron and contains the lowest percentage of carbon viz. 0.12 to 0.25% and 0.3% of impurities like S, P, Si and Mn.

