
In an organic reaction, the organic molecule (also referred as a substrate) reacts with an appropriate attacking reagent and leads to the formation of one or more intermediate(s) and finally product(s)
The general reaction is depicted as follows:
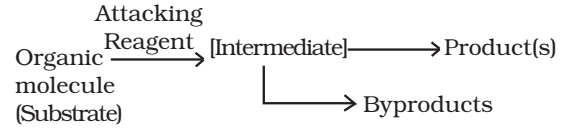
A sequential account of each step, describing details of electron movement, energetics during bond cleavage and bond formation, and the rates of transformation of reactants into products (kinetics) is referred to as reaction mechanism.
Fission as a Covalent Bond:
A covalent bond can get cleaved either by: (i) heterolytic cleavage, or by (ii) homolytic cleavage.
Heterolytic Fission:
In this, the bond breaks in such a fashion that the shared pair of electrons goes with one of the fragments.

Carbon bearing a positive charge is called carbocation and carbon bearing negative charge is called carbanion.
Heterolytic fission generally takes place in polar covalent molecules but in non-polar molecules, it takes place in the presence of catalyst like AiCI3 (anhy.), FeCl3 (anhy.) etc.
Homolytic Fission:
In this, one of the electrons of the shared pair in a covalent bond goes with each of the bonded atoms. the neutral chemical species thus formed, is called free radical. Generally, homolytic fission takes place in non-polar, covalent molecules in the presence of sunlight or high temperature.
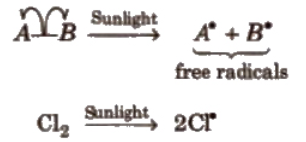
Free radicals are highly reactive. Neutral and electron deficient species.
Attacking Reagent:
These are of two types: (i) Electrophiles or Electrophilic Reagents and (ii) Nucleophiles or Nucleophilic Reagents.
Electrophiles or Electrophilic Reagents:
These are electron deficient species i.e., behave as Lewis acids. The following species behave as electrophiles:
- All non-metal cations and metal cations which have vacant d-orbitals.
![]()
- Lewis acids (incomplete octet) e.g., BF3, FeCl3 (anhydrous), AlCl3 (anhydrous), etc.
- Non-metal (acidic) oxides e.g., CO2, SO2 etc.
Nucleophiles or Nucleophilic Reagents:
These are electron rich species i.e, behave as Lewis bases. These attack at electron deficient area. The following species behave as nucleophiles:
- All anions e.g.,
![]()
- Lewis bases e.g.,
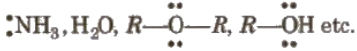
- Benzene, alkenes etc.
Nucleophilicity order is
![]()
In case of same nucleophilic site, nucleophilicity parallels basicity i.e., as the basicity increases, nucleophilicity also increases.
If nucleophilic sites (or attacking atoms) are different nucleophilicity varies inversely with electronegativity)

