
In p-block elements, the last electron enters in the outermost p-orbital. There are six groups of p-block elements in the Periodic Table, numbering from 13 to 18. Their valence shell electronic configuration is ns2np1-6 (except for He).

Group 13 Elements
It is also called the boron family. It includes B, Al, Ga, In, Tl. AI is the most abundant metal and third most abundant element in the earth’s crust.
Physical Properties of Group 13 Elements
Electronic configuration:
Elements of group 13 have the general outer electronic configuration of ns2np1.
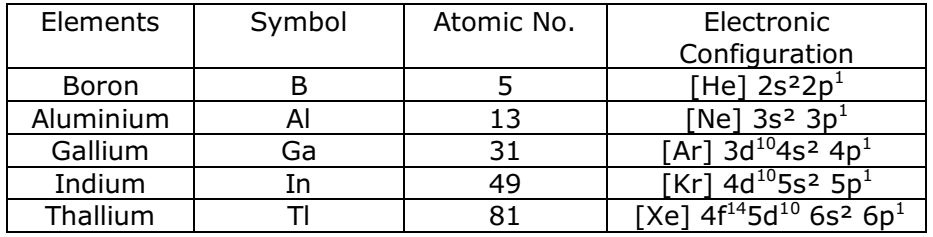
Atomic Radii:
Atomic radii increases down the group but not regularly.
Ionic Radii:
Ionic radii regularly increases from B3+ to Tl3+.
Density:
It increases regularly on moving down the group from B to Tl.
Melting and Boiling Point:
The melting point and boiling point of group 13 elements are much higher than those of group 2 elements. The melting point decreases from B to Ga and then increases, due to structural changes in the elements
Ionization enthalpy:
Ionization enthalpy decreases down the group but not regularly.
Electronegativity:
Down the group, electronegativity first decreases from B to Al and the increases marginally.
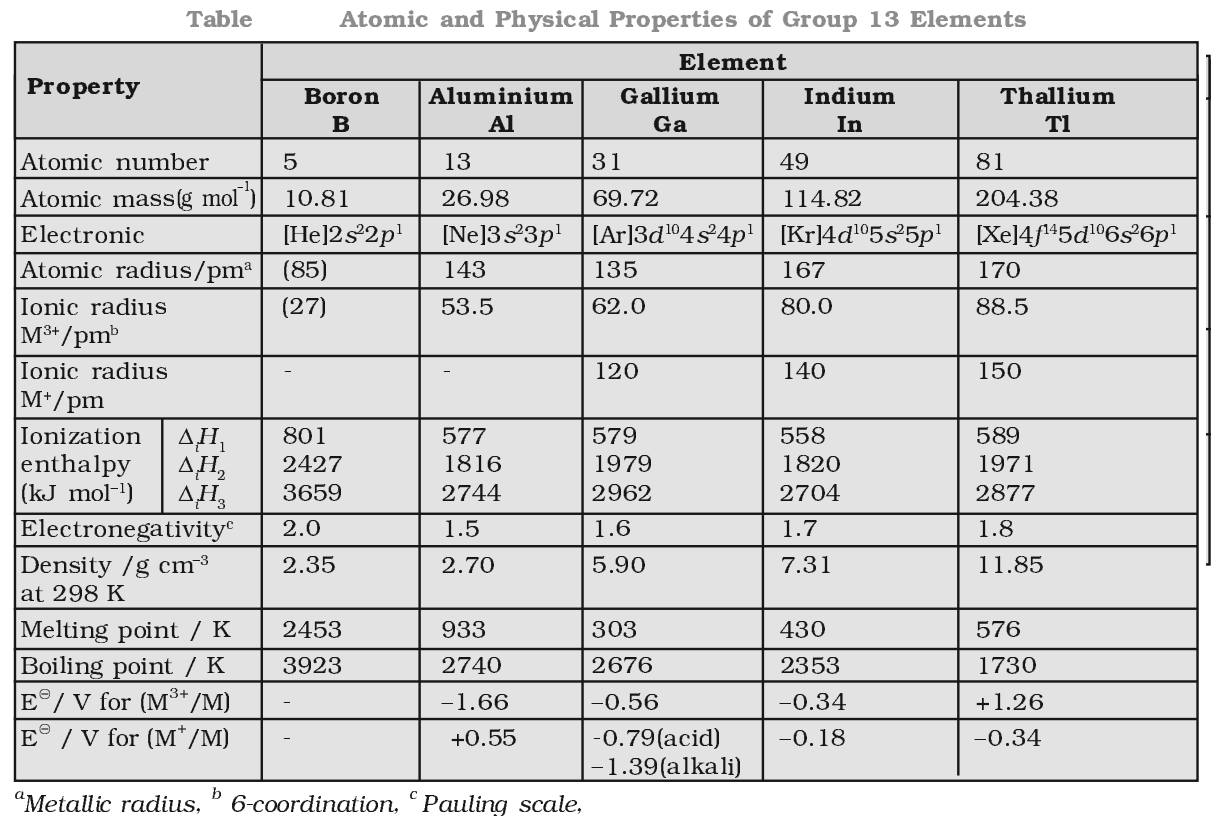
Oxidation state:
B and Al show an oxidation state of +3 only while Ga, In and TJ exhibit oxidation states of both +1 and +3. As we move down in the group 13. due to inert pair effect, the tendency to exhibit +3 oxidation state decreases and the tendency to attain +1 oxidation state increases.
Stability of +1 oxidation state follows the order Ga < In < Tl.
Chemical Properties of Group 13 Elements
Reactivity towards air:
Crystalline boron is unreactive whereas amorphous boron is reactive. It reacts with air at 700oC as follows
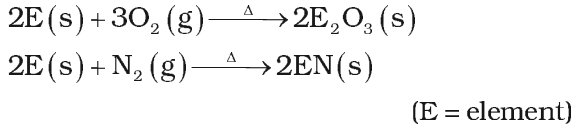
Reactivity towards acids and alkalies:
Boron does not react with acids and alkalies even at moderate temperature; but aluminium dissolves in mineral acids and aqueous alkalies and thus shows amphoteric character.
Aluminimum dissolved in dilute HCl and liberates dihydrogen.
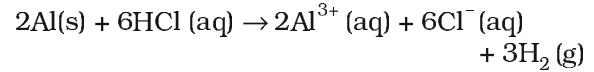
However, concentrated nitric acid renders aluminium passive by forming protective oxide layer on the surface. Aluminium also reacts with aqueous alkali and liberates dihydrogen.
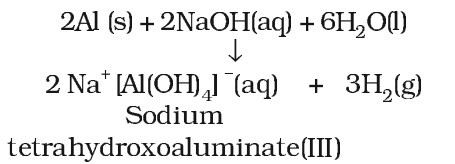
Reactivity towards halogens:
These elements react with halogens to form trihalides (except TlI3).
![]()

