
Physical Properties of Group 14 Elements
Electronic Configuration:
Elements of group 14 have the general outer electronic configuration of ns2np2.
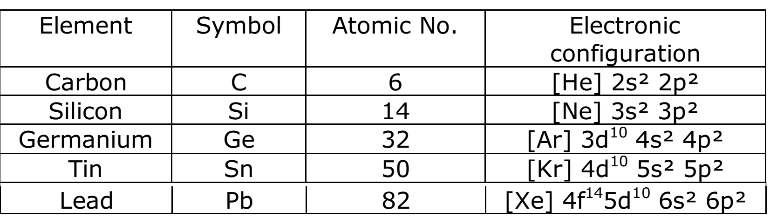
Covalent Radius:
There is a considerable increase in covalent radius from C to Si, thereafter from Si to Pb a small increase in radius is observed. This is due to the presence of completely filled d and f orbitals in heavier members.
Ionization Enthalpy:
Ionization enthalpy decreases down the group but not regularly. Small decreases in ∆iH from Si to Ge to Sn and slight increase in ∆iH from Sn to Pb is the consequence of poor shielding effect of intervening d and f orbitals and increase in size of the atom.
Electronegativity:
Due to small size, the elements of this group are slightly more electronegativity than group 13 element. The electronegativity values for elements from Si to Pb are almost the same.
Density:
Density increases with increase atomic number due to increase in mass per unit volume down the group.
Melting and Boiling Point:
The m.p and b.p decrease from carbon to lead but carbon and silicon have very high m.p and b.p due to their giant structure.
Oxidation state:
They exhibit +2 and +4 oxidation state. The compounds of Pb in +4 oxidation state are powerful oxidising agents since, +2 oxidation state of Pb is more stable due to inert pair effect.
The compounds in +2 oxidation state are ionic in nature and in +4 oxidation state are covalent in nature (Acc. To Fajan’s rule).
Chemical Properties of Group 14 Element
Reactivity towards oxygen:
All members when heated in oxygen form oxides. There are mainly two types of oxides, i.e., monoxide and dioxide of formula MO and MO2 respectively.
- mono-oxide of the type MO. E.g., CO (neutral) and SiO, GeO, (all basic).
- Di-oxide of the type of MO2. E.g.,

CO2 is linear gas at ordinary temperature. Solid CO2 is know as dry ice or drikold.
Reactivity towards water:
Carbon, silicon and germanium are not affected by water. Tin decomposes steam to form dioxide and dihydrogen gas. Lead is unaffected by water, probably because of a protective oxide film formation.
Reactivity towards halogen:
Halides: All the elements give tetrahedral and covalent halides of the type MX4 except PbBr4 and PbI4.
- Thermal Stability
![]()
- Order of thermal stability with common metals
![]()
- Except CX4 other tetrahalides can hydrolysed due to the presence of vacant d-orbitals.
![]()
Ease of hydrolysis:
![]()
Except C, other element form dihalides of the type MX2 which are more ionic and have higher m.p and b.p, e.g., SnCl2 is a solid whereas SnCl4 is a liquid at room temperature.
- SnCl2. 5H2O is called bitter of tin and is used as a mordant in dyeing.

