Group 16 Element
- Oxygen, sulphur, selenium, tellurium and polonium constitute Group 16 of the periodic table.
- This is sometimes known as a group of chalcogens.
Occurrence
- Oxygen is the most abundant of all the elements on the earth. Oxygen forms about 46.6% by mass of earth’s crust. Dry air contains 20.946% oxygen by volume.
- However, the abundance of sulphur in the earth’s crust is only 0.03-0.1%. Combined sulphur exists primarily as sulphates such as gypsum CaSO4.2H2O, Epsom salt MgSO4 .7H2O, baryta BaSO4 and sulphides such as galena PBS, zinc blende ZnS, copper pyrites CuFeS2. Traces of sulphur occur as hydrogen sulphide in volcanoes.
- Selenium and tellurium are also found as metal selenides and tellurides in sulphide ores. Polonium occurs in nature as a decay product of thorium and uranium minerals.
Electronic Configuration
The elements of group 16 have six electrons in the outermost shell and have ns2 np4 general electronic configuration.
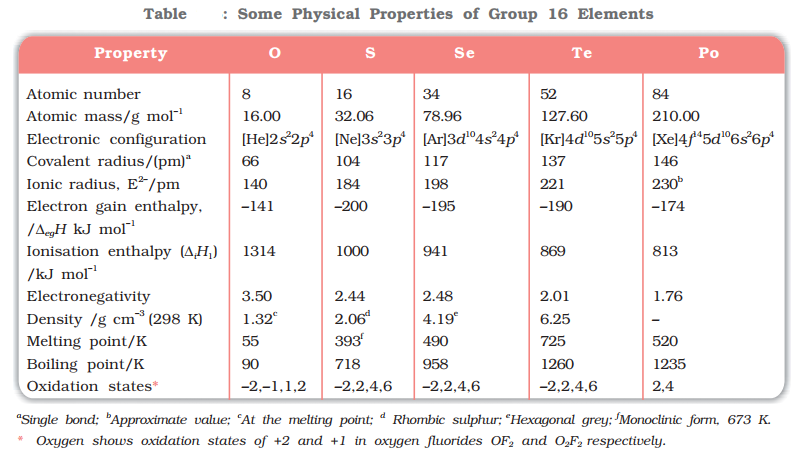
Atomic and Ionic Radii
Due to increase in the number of shells, atomic and ionic radii increase from top to bottom in the group. The size of oxygen atoms is, however, exceptionally small.
Ionisation Enthalpy
Ionisation enthalpy decrease down the group. It is due to increase in size. However, the element of this group has lower ionisation enthalpy values compared to those of group 15 in the corresponding periods. This is due to the fact that group 15 element have extra stable half-filled p orbitals electronic configurations.
Electron Gain Enthalpy
Because of the compact nature of oxygen atom, it has less negative electron gain enthalpy than sulphur. However, from sulphur onwards, the value again becomes less negative up to polonium.
Electronegativity
Next to fluorine, oxygen has the highest electronegativity value amongst the elements. Within the group, electronegativity decreases with an increase in atomic number. This implies that the metallic character increase from oxygen to polonium.
Melting and Boiling Point
- The melting and boiling points increase with an increase in atomic number down the group.
- The larger difference between the melting and boiling points of oxygen and sulphur may be explained on the basis of their atomicity.
- Oxygen exists as diatomic molecules (O2)
- Whereas sulphur exists as polyatomic molecule (S8).
Metallic Character
- Oxygen and sulphur are non-metal
- Selenium and tellurium metalloids.
- Polonium is a metal.
- Polonium is radioactive and is short-lived (Half-life 13.8 days). All these element exhibit allotropies.