
Hybridisation
Hybridisation is the process of intermixing of the orbitals of slightly different energies so as to redistribute their energies resulting in the formation of new set of orbitals of equivalent and shape.
Salient Features of Hybridisation:
- Orbitals with almost equal energy take part in the hybridisation.
- Number of hybrid orbital produced is equal to the number of atomic orbitals mixed,
- Geometry of a covalent molecule can be indicated by the type of hybridisation.
- The hybrid orbitals are more effective in forming stable bonds than the pure atomic orbitals.
Conditions necessary for hybridisation:
- Orbitals of valence shell take part in the hybridisation.
- Orbitals involved in hybridisation should have almost equal energy.
- Promotion of electron is not necessary condition prior to hybridisation.
- In some cases, filled orbitals of valence shell also take part in hybridisation.
Types of Hybridisation:
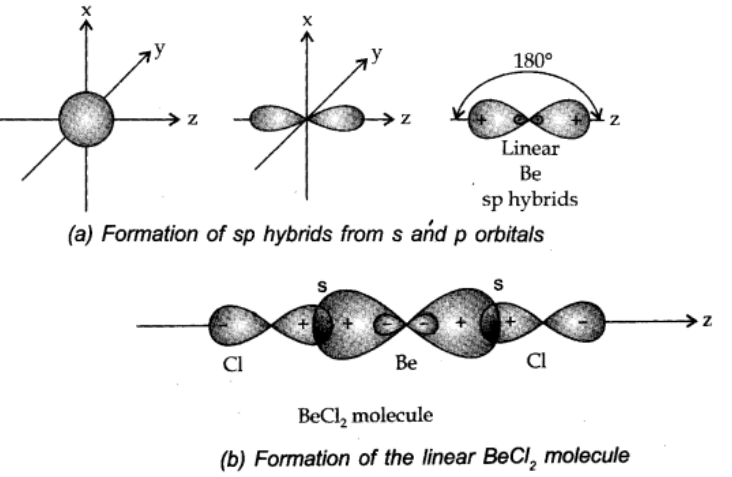
sp hybridisation:
When one s and p-orbital hybridize to form two equivalent orbitals, the orbital is as sp hybrid orbital, and the type of hybridisation is called sp hybridisation.
Each of the hybrid orbitals formed has 50% s-character and 50% p-character. This type of hybridisation is also known as diagonal hybridisation.
Examples: All compounds of carbon containing C≡C triple bond like acetylene (C2H2), BeCl2, BeF2, BeH2 etc.
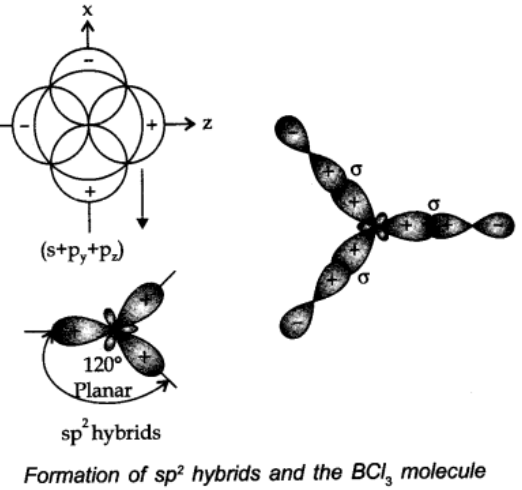
sp2 hybridisation:
In this type, one s and two p-orbitals hybridize to form three equivalent sp2 hybridized orbitals.
All the three hybrid orbitals remain in the same plane making an angle of 1200.
Example: A few compounds in which sp2 hybridisation takes place are BF3, BH3, BCl3 carbon compounds containing double bond etc.
sp3 hybridisation:
In this type, one s and three p-orbitals in the valence shell of an atom get hybridized to form four equivalent hybrid orbitals. There is 25% s-character and 75% p-character in each sp3 hybrid orbital. The four sp3 orbitals are directed towards four corners of the tetrahedron.
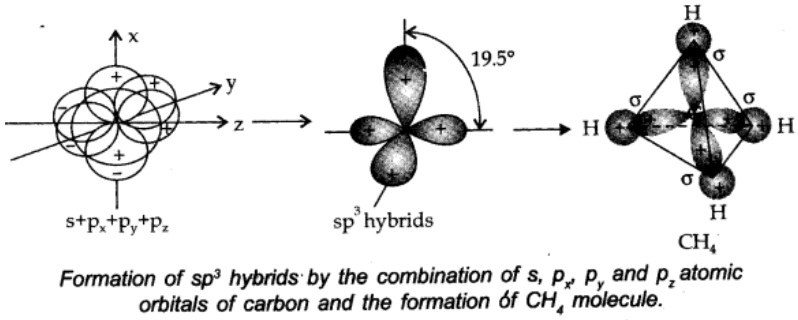
The angle between sp3 hybrid orbitals is 109.50.
A compound in which sp3 hybridisation occurs is, (CH4). The structure of NH2 and H2o molecules can also be explained with the help of sp3 hybridisation.

