Orbit Overlap Concept
According to orbital overlap concept, the formation of a covalent bond between two atoms results by pairing of electrons present in the valence shell having opposite spins.
Types of Orbital Overlap
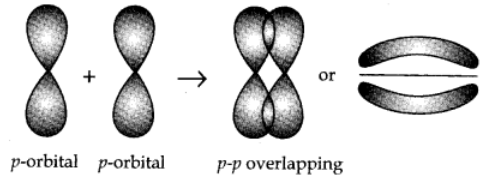
Depending upon the type of overlapping, the covalent bonds are of two types, known as sigma (σ) and pi (π) bond.
Sigma (σ bond):
Sigma bond is formed by the end to end (head-on) overlap of bonding orbitals along the internuclear axis.
The axial overlap involving these orbitals is of three types:
s-s overlapping:
In this case, there is overlap of two half-filled s-orbitals along the internuclear axis as shown below:

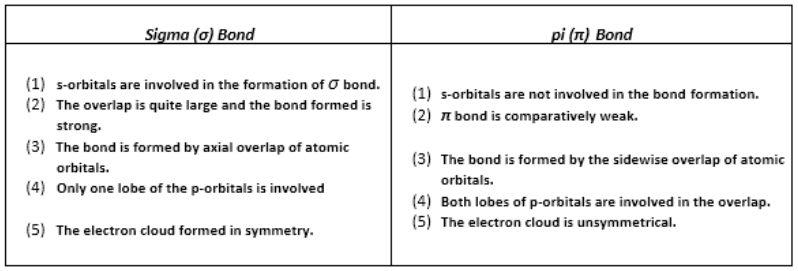
s-p overlapping:
This type of overlapping occurs between half-filled s-orbitals of one atom and half-filled p-orbitals of another atoms.
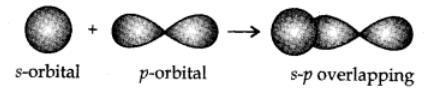
p-p overlapping:
This type of overlapping takes place between half filled p-orbitals of the two approaching atoms.

pi (π bond):
π bond is formed by the atomic orbitals when they overlap in such a way that their axis remains parallel to each other and perpendicular to the internuclear axis. The orbital formed is due to lateral overlapping or side wise overlapping.
Strength of Sigma and pi Bonds
Sigma bond (σ bond) is formed by the axial overlapping of the atomic orbitals while the π-bond is formed by side wise overlapping. Since axial overlapping is greater as compared to side wise. Thus, the sigma bond is said to be stronger bond in comparison to a π-bond.
The distinction between sigma and π-bonds