
Preparation of Hydrogen Chloride:
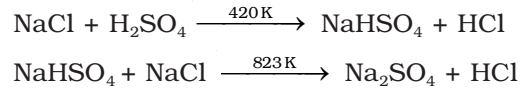
It is prepared by heating sodium chloride with concentrated sulphuric acid.
Properties of Hydrogen Chloride:
- HCl is a colourless gas with a pungent odour.
- It is extremely soluble in water
- It decomposes salts of weaker acids.
- It has a pungent suffocating smell.
- The gas fumes in moist air.
- It is heavier than air (vapour density is 18.25). it is easily liquefied to a colourless liquid (b.p. 189 K) and freezes to a white crystalline solid (f.p. 159 K).
- Its b.p is 189 K and m.p. is 159 K.
Uses of Hydrogen Chloride:
- Hydrogen chloride is used in medicine and as a laboratory reagent.
- It is used in the manufacture of chlorine, NH4Cl and glucose.

