
Laws of Chemical Combinations
The combination of elements to form compounds is governed by the following five basic laws.
- Law of conservative of mass
- Law of Definite Proportions
- Law of Multiple Proportions
- Gay Lussac’s Law of Gaseous Volumes
- Avogadro Law
Law of Conservation of mass (Lavoisier, 1774)
This law states that during any physical or chemical change, the total mass of the products is equal to the total mass of reactants. It does not hold good for nuclear reactions.
Law of definite proportions or Law of constant composition (Proust, 1799)
This law states that “A chemical compound always consists of the same elements combined together in the same ratio, irrespective of the method of preparation or the source from where it is taken”.
Law of Multiple Proportions (Dalton 1803)
This law states that “When two elements combine to form two or more compounds, then the different masses of one element, which combine with a fixed mass of the other, bear a simple ratio to one another”.
Gay Lussac’s Law of Gaseous Volumes (Gay Lussac 1808)
According to this law when gases combine or are produced in chemical reaction they do so in a simple ratio by volume provided all gases are at the same temperature and pressure.
Example:
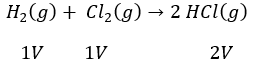
All reactions and products have simple ratio 1:1:2
Avogadro Law (Avogadro)
According to this law equal volumes of gases at the same temperature and pressure should contain the equal number of molecules.

