
Dalton’s Atomic Theory
The origin of the idea that matter is composed of small indivisible particles called ‘a-Tomio’ (meaning — indivisible). In 1808, Dalton published ‘A New System of Chemical Philosophy’ in which he proposed the following:
- Matter consists of indivisible atoms.
- All the atoms of a given element have identical properties including identical mass. Atoms of different elements differ in mass.
- Compounds are formed when atoms of different elements combine in a fixed ratio.
- Chemical reactions involve reorganization of atoms. These are neither created nor destroyed in a chemical reaction. Dalton’s theory could explain the laws of chemical combination.
Atomic Mass
- The atomic mass or the mass of an atom is actually very-very small because atoms are extremely small.
- The atomic mass of an element is the number of times an atom of that element is heavier than an atom of carbon taken as 12.
- It may be noted that the atomic masses as obtained above are the relative atomic masses and not the actual masses of the atoms.
- One atomic mass unit (amu) is equal to 1/12th of the mass of an atom of carbon-12 isotope. It is also known as unified mass.
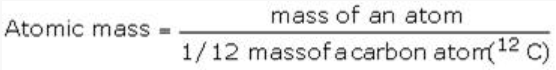
1 amu = 1.66056 x 10-24 g
- Today ‘amu’ has been replaced by ‘u’ which is known as unified mass.
Average Atomic Mass
- Most of the elements exist as isotopes which are different atoms of the same element with different mass numbers and the same atomic number.
- Therefore, the atomic mass of an element must be its average atomic mass.
- And it may be defined as the average relative mass of an atom of an element as compared to the mass of carbon atoms (C-12) taken as 12w.
Molecular Mass
- Molecular mass is the sum of atomic masses of the elements present in a molecule.
- It is obtained by multiplying the atomic mass of each element by the number of its atoms and adding them together.
- For example,
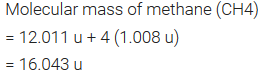
Formula Mass
Sum of atomic masses of the elements present in one formula unit of a compound. It is used for the ionic compounds.
Mole Concept
- Mole is defined as the amount of a substance, which contains the same number of chemical units (atoms, molecules, ions or electrons) as there are atoms in exactly 12 grams of pure carbon-12.
- A mole represents a collection of 6.022 x 1023 (Avogradro’s number) chemical units.
- The mass of one mole of a substance in grams is called its molar mass.
Molar Volume
- The volume occupied by one mole of any substance is called its molar volume.
- It is denoted by Vm.
- One mole of all gaseous substances at 273 k and 1 atm pressure occupies a volume equal to 22.4 litre or 22,400 ml.
- The unit of molar volume is litre per mol or millilitre per mol.
Percentage Composition
The mass percentage of each constituent elements present in any compound is called its percentage composition.
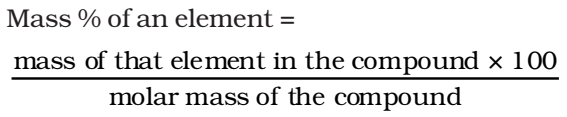
Empirical Formula for molecular formula
An empirical formula represents the simplest whole number ratio of various atoms present in a compound.
Example:
- The formula of hydrogen peroxide is H2O2.
- In order to express its empirical formula, we have to take out a common factor 2. The simplest whole number ratio of the atoms is 1:1 and the empirical formula is HO.
- Similarly, the formula of glucose is C6H12O6.
- In order to get the simplest whole number of the atoms, Common factor = 6
The ratio is = 1:2:1
- The empirical formula of glucose =CH2O
Molecular formula
The molecular formula shows the exact number of different types of atoms present in a molecule od a compound.
Example:
- The molecular formula of hydrogen peroxide = H2O2 and Glucose = C6H12O6
- Molecular formula = n x Empirical formula
- Where n is the common factor and also called multiplying factor. The value of n may be 1, 2, 3, 4, 5, 6 etc.
- In case n is 1, the Molecular formula of a compound = Empirical formula of the compound.

