
Organic compounds are the hydrocarbons and their derivatives and organic chemistry is that branch of chemistry that deals with the study of these compounds.
Tetravalency of carbon
The atomic number of Carbon is 6 and its electronic configuration is 2,4 i.e. it has 4 valence electrons. Thus, carbon is always tetra-covalent, i.e. it forms 4 covalent bonds with other atoms
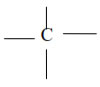
Due to tetravalency of carbon, it has a tetrahedron shape.
The shapes of carbon Compounds:
In organic or carbon compounds, s and p orbitals are involved in hybridisation. This leads to y three types of hybridisation which are sp3(in alkanes) – Tetrahedral in shape sp2(in alkenes) – Planar structure sp(in alkynes) – Linear molecule.
Catenation
It is the tendency of self-combination and is maximum in carbon. A carbon atom can combine with other carbon atoms by single, double or triple bonds. Thus, it forms more compounds than the others.

