
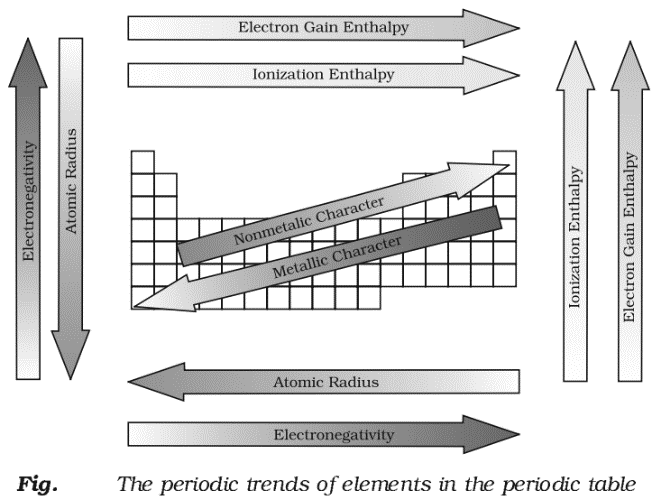
Trends in Physical Properties
There are numerous physical properties of elements such as melting and boiling points, heats of fusion and vaporization, energy of atomization, etc. which show periodic variations.
Atomic Radius:
It is defined as the distance from the centre of the nucleus to the nucleus to the outermost shell containing the electrons. Depending upon whether an element is a non-metal or a metal, three different types of atomic radii are used. These are:
- Covalent Radius: The half of the distance between the nuclei of two identical atoms joined by single covalent bond in a molecule is known as covalent radius.
- Van der waal’s radius: It is half of the internuclear distance between adjacent atoms of the two neighboring molecules in the solid state.
- Metallic Radius (Crystal radius): It is one-half of the distance between the nuclei of two adjacent metal atoms in the metallic crystal lattice.
- The order of radii is
Vander Waal’s radius > Metallic radius > Covalent radius
Ionic Radius:
The effective distance from the centre of the nucleus of an ion upto which it has an influence on its electron cloud is called its ionic radius. A cation is smaller but the anion is larger than the parent atom. In case of isoelectronic species, the cation with greater positive charge has smaller radius but anion with greater negative charge has the larger radii.
Ionization Enthalpy:
The minimum amount of energy required to remove the electron from the outermost orbit of an isolated atom in the gaseous state is known as ionization energy.
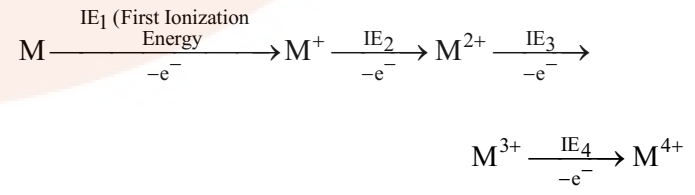
IE1, IE2, IE3 and IE4 are successive ionization energies.
Variation of Ionization Energy in Periodic Table.
Various factors with which IE vanes are:
- Atomic size: varies inversely
- Screening effect: varies inversely
- Nuclear charge: varies directly
Electron Gain Enthalpy:
When an electron is added to a neutral gaseous atom (X) to convert it into a negative ion, the enthalpy change accompanying the process is defined as the Electron Gain E n t h a l p y ( egH). Electron gain enthalpy provides a measure of the ease with which an atom adds an electron to form anion as represented by
![]()
Various factors with which electron gain enthalpy varies are:
- Atomic size: varies directly
- Nuclear charge: varies directly
Along a period, electron gain enthalpy becomes more and more negative while on moving down the group, it become less negative.
Noble gases have positive electron gain enthalpies.
Halogen have maximum value of ∆Heg with in a period due to smallest atomic size.
F and O atom have small size and high charge density, therefore have lower electron gain enthalpy, than Cl and S respectively
Cl > F ; S > O
Elements having half-filled and fully-filled orbitals exhibit more stability. Therefore, electron gain enthalpy will be low for such elements.
Electron gain enthalpy can be measured by Born-Haber cycle and elements with high ∆Heg are good oxidising agent.
Electronegativity (EN):
It is defined as the tendency of an atom to attract the shared electron pair towards itself in a covalent bond. Various factors with which electronegativity varies are:
- Atomic size: varies inversely
- Charge on the ion: varies directly, e.g., Li < Li+, Fe2+ < Fe3+
- Hybridisation: (Electronegativity &infi; s-character in the hybrid orbital)
Electronegativity of carbon atom = C2H6 < C2H4 < C2H2
In periods as we move from left to right electronegativity increases, while in the groups electronegativity decreases down the group.
For noble gases, its value is taken as zero.
Electronegativity helps to predict the polarity of bonds and dipole moment of molecules.
Electronegativity order of some elements (on pauling scale) is
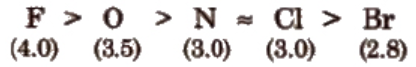
Mulliken Scale
![]()
Pauling Scale
The difference in electronegativity of two atoms A and B is given by the relationship.
![]()
Where,
![]()
(∆ is known as resonance energy.)
EA-B, EA-A and EB-B represent bond dissociation energies of the bonds A-B, A-A and B-B respectively.
Allred and Rochow method
![]()
Valency:
- It is defined as the combining capacity of the element. The valency of an element is related to the electronic configuration of its atom and usually determined by electrons present in the valence shell,
- On moving along a period from left to right, valency increases from 1 to 4 and then decreases to zero (for noble gases) while on moving down a group the valency remains the same.
- Transition metals exhibit variable valency because they can use electron rom outer as well as penultimate shell.
Chemical Reactivity
- Reactivity of metal increases with decreases in IE, electronegativity and increase in atomic size as well as electropositive character.
- Reactivity of non-metals increases with increase in electronegativity as well as electron gain enthalpy and the decrease in atomic radii.
Melting and Boiling Points
- On moving down the group, the melting point and boiling point for metallic elements go on decreasing due to the decreasing forces of attraction. However, for non-metals, melting point and boiling point generally increase down the group.
- Tungsten (W) has highest m.p. (3683 K) among metals, carbon (diamond) has the highest m.p, (4000 K) among non-metals.
- Li metal has minimum density while iridium (Ir) metal has maximum density.
Electropositivity or Metallic Character
- The tendency of an atom of the element to lose valence electrons and form positive ion is called electropositivity.
- Greater the electropositive character, greater is the metallic character.
- Electropositive character decreases on moving across the period and increases on moving down the group.
- Alkali metals are the most electropositive and halogens are the least electropositive element in their respective period.
- Basic nature of oxides of metallic character, i.e., it also decreases along a period and increases down the group.

