
Quantum numbers
There are a set of four quantum numbers which specify the energy, size, shape and orientation of an orbital. To specify an orbital only three quantum numbers are required while to specify an electron all four quantum numbers are required.
Principal Quantum Number (n) (Niels Bohr)
It identifies shell, determines sizes and energy of orbitals.
It is denoted by 11. It tells us about the main shell in which electron resides. It also gives an idea about the energy of the shell and the average distance of the electron from the nucleus.
Value of n = any integer.
Azimuthal Quantum Number (Sommerfeld)
It is denoted by I. it tells about the number of subshells (s, p, d, f) in any main shell. It also represents the angular momentum of an electron and shapes of subshells. The orbital angular momentum of an
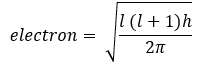
Value of l = 0 to n-1.
I = 0 for s
I = 2 for d
I = 1 for P
I = 3 for f
Number of subshells in main energy level =n.
Magnetic Quantum Number or Magnetic orbital quantum number (ml)
It gives information about the spatial orientation of the orbital with respect to a standard set of co-ordinate axis. For any sub-shell (defined by ‘l’ value) 2l+1 values of ml are possible. For each value of l, ml = -1,
-(1-1), -(1-2) …. 0, 1… (1-2), (1-1),1
Electron Spin Quantum Number (ms)
It refers to the orientation of the spin of the electron. It can have two values +1/2 and -1/2.
+1/2 identifies the clockwise spin.
-1/2 identifies the anti-clockwise spin.

