
Atom
Atom is the smallest indivisible particle of the matter. Atom is made of electron, proton and neutrons.
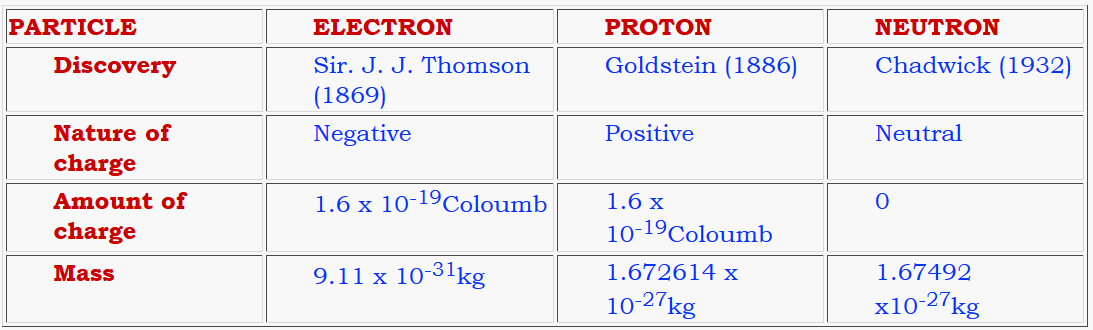
Electron was discovered using cathode ray discharge tube experiment.
The nucleus was discovered by Rutherford in 1911.
Cathode ray discharge tube experiment: A cathode ray discharge tube made of glass is taken with two electrodes. At very low pressure and high voltage, current starts flowing through a stream of particles moving in the tube from cathode to anode. These rays were called cathode rays. When a perforated anode was taken, the cathode rays struck the other end of the glass tube at the fluorescent coating and a bright spot on the coating was developed.
Thomson model of Atom
Sir J. J. Thomson, who discovered the electron, was the first to suggest a model of atomic structure.
- All atoms contain electrons.
- The atom as a whole is neutral. The total positive charge and total negative charge must be equal.
- He visualized all the positive charge of the atom as being spread out uniformly throughout a sphere of atomic dimensions (i.e. approx. 10-10 m in diameter).
- The electrons were smaller particles together carrying a negative charge, equal to the positive charge in the atom.
- They were studded in the atom like plums in a pudding. The charge distribution was such, that it gave the most stable arrangement.
- This model of the atom was often called the plum – pudding model. Also, the raisin pudding model or watermelon model.

Drawbacks
Though the model was able to explain the overall neutrality of the atom, it could not satisfactorily explain the results of scattering experiments carried out by Rutherford.
Rutherford’s Model
Rutherford’s α-particle Scattering Experiment
Rutherford conducted – particles scattering experiments in 1909. In this experiment, a very thin foil of gold (0.004nm) is bombarded by a fine stream of alpha particles. A fluorescent screen (ZnS) is placed behind the gold foil, where points were recorded which were emerging from -particles. Polonium was used as the source of -particles.
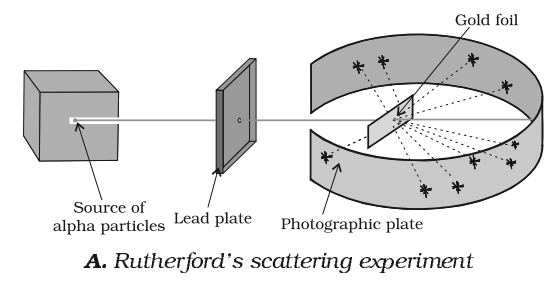
Observations
Rutherford carried out a number of experiments, involving α-particles by a very thin foil of gold. Observations were:
- Most of the particles (99%) pass through it, without any deviation or deflection.
- Some of the particles were deflected through small angles.
- Very few particles were deflected by large angles and occasionally a particle got deflected by 1800.
Conclusions
- An atom must be extremely hollow and must consist of mostly empty space because most of the particles passed through it without any deflection.
- Very few particles were deflected to a large extent. This indicates that:
- Electrons because of their negative charge and very low mass cannot deflect heavy and positively charged -particles.
- There must be a very heavy and positively charged body in the atom i.e. nucleus which does not permit the passage of positively charged particles.
- Because, the number of particles which undergo deflection of 180º, is very small, therefore the volume of a positively charged body must be an extremely small fraction of the total volume of the atom. This positively charged body must be at the centre of the atom which is called a nucleus.
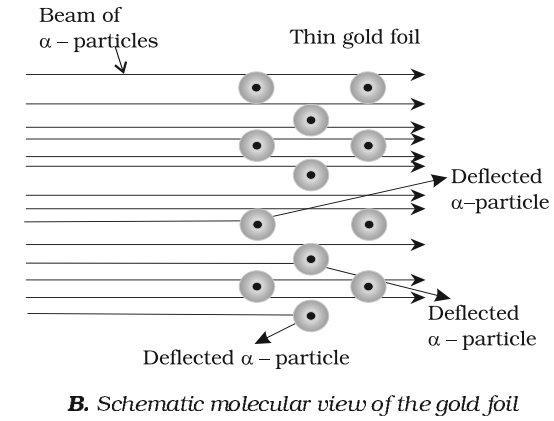
It has been found that radius of the atom is of the order of 10-10m while the radius of the nucleus is of the order of 10-15m Thus if a cricket ball represents a nucleus, then the radius of the atom would be about 5 km.
Rutherford’s nuclear atomic model
On the basis of the above observation and conclusion, Rutherford proposed the nuclear model of the atom (after the discovery of protons). According to this model:
- The positive charge and most of the mass of the atom was densely concentrated in the extremely small region. This very small portion of the atom was called nucleus by Rutherford.
- The nucleus is surrounded by electrons that move around the nucleus with a very high speed in circular paths called orbits. Thus, Rutherford’s model of atom resembles the solar system in which the nucleus plays the role of the sun and the electrons that of revolving planets.
- Electrons and the nucleus are held together by electrostatic forces of attraction.
Drawbacks
- It cannot explain the stability of an atom on the basis of classical mechanics and electromagnetic theory.
- If the electrons were stationary, then attraction between the dense nucleus and the electrons would pull the electrons towards the nucleus. Thus, it cannot explain the stability of an atom.
- According to classical mechanics, any charged body in motion under the influence of attractive forces should radiate energy continuously. If this is so, the electron will follow a spiral path and finally fall into the nucleus and the structure would collapse. This behaviour is never observed.
- It says nothing about the electronic structure of atoms i.e. how the electrons are distributed around the nucleus and what are the energies of these electrons.
- It cannot explain the atomic spectra.

