- These hydrocarbons are also known as ‘arenes’. Since most of them possess pleasant odour (Greek; aroma meaning pleasant smelling), the class of compounds was named as ‘aromatic compounds’.
- Aromatic compounds containing benzene ring are known as benzenoids and those not containing a benzene ring are known as non-benzenoids. Some example of arenes are given below:
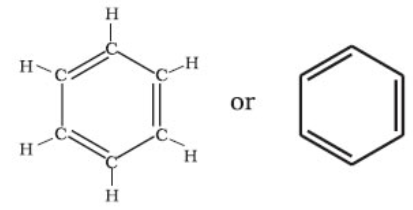
- Structure of Benzene -Kekule` structure.
Nomenclature and Isomerism
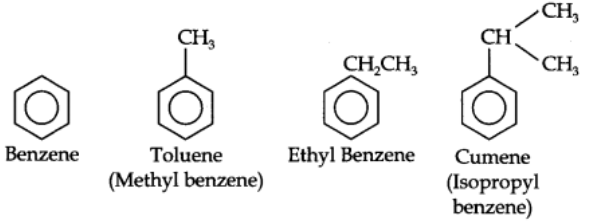
Benzene and its homologous are generally called by their common names which are accepted by the IUPAC system. The homologous of benzene having a single alkyl group are named as Alkyl benzenes.
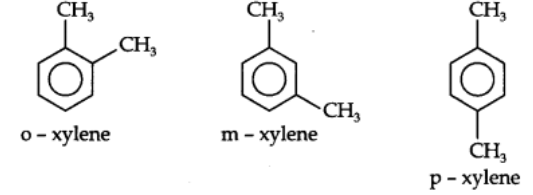
Dimethylbenzenes have the common name Xylenes. The three isomeric xylenes are
Structure of Benzene
By elemental analysis, it is found that molecular formula of benzene is C6H6. This indicates that benzene is a highly unsaturated compound. In 1865, Kekule gave the cyclic planar structure of benzene with six carbons with alternate double and single bonds.
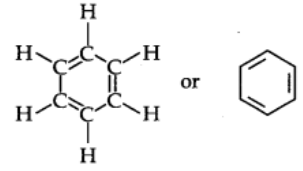
The Kekule structure indicates the possibility of two isomeric 1,2-dibromobenzenes. In one of the isomers, the bromine atoms are attached to the doubly bonded carbon atoms whereas in the other they are attached to the singly bonded carbon.
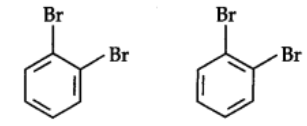
In fact, only one ortho-dibromo benzene could be prepared.
To overcome this problem Kekule suggested that benzene was a mixture of two forms.
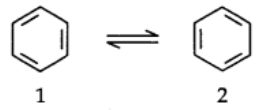
Failure of Kekule’s structure:
Kekule structure of benzene failed to explain the unique stability and its preference to substitution reaction than addition reactions.
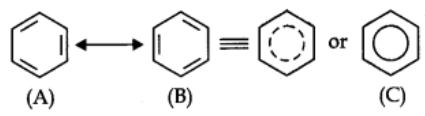
Resonance Structure of Benzene:
The phenomenon in which two or more structures can be written for a substance which involve identical positions of atoms is called resonance.
In benzene’s Kekule’s structures (1) and (2) represent the resonance structures.
Actual structure – of the molecule is represented by hybrid of these two structures.
Orbital structure of benzene
- All six carbon atoms in benzene are sp2 hybridized.
- The sp2 hybrid orbitals overlap with each other and with s orbitals of the six hydrogen atoms forming C—C and C—H σ-bonds.
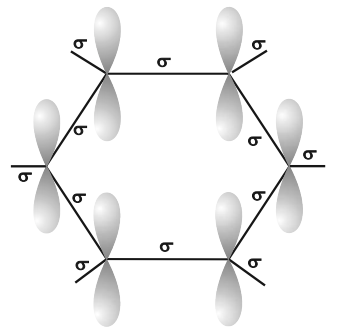
- X-Ray diffraction data indicates that benzene is a planar molecule. The data indicates that all the six C—C bond length are of the same order (139 pm) which is intermediate between (C—C) single bond (154 pm) and C—C double bond (133 pm).
- Thus, the presence of pure double bond in benzene gives the idea of reductance of benzene to show addition reaction under normal condition. The is, it explains the unusual behaviour of benzene.
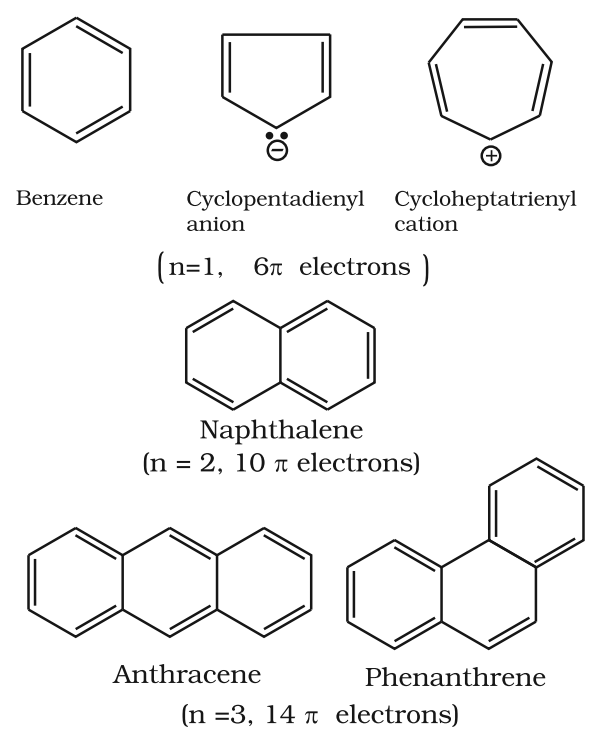
Aromaticity
- It is a property of the sp2 hybridized planar rings in which the p orbitals allow cyclic delocalization of π electrons.
- An aromatic compound is cyclic and planar.
- Each atom in an aromatic ring has a p orbital. These p orbitals must be parallel so that a continuous overlap is possible around the ring.
- The cyclic π molecular orbital (electron cloud) formed by overlap of p orbitals must contain (4n + 2) π electrons.
Where n = integer (0, 1, 2, 3, etc.). This is known as Huckel rule.
Some Examples of Atomic Compounds are given below:
Preparation of Benzene
Benzene is commercially isolated from coal tar. However, there are some synthetic methods which is applied in the laboratory for the preparation of benzene.
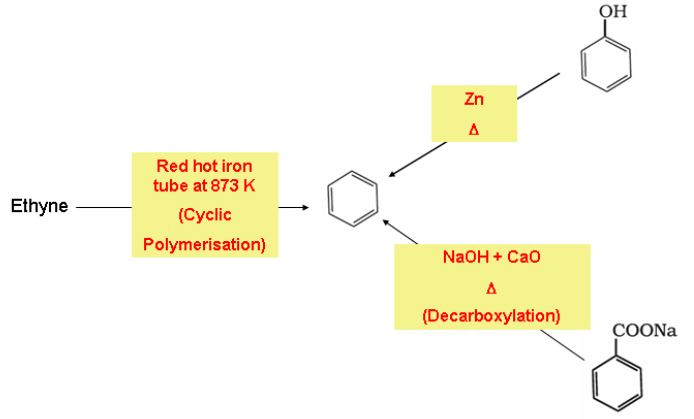
- Cyclic polymerisation of ethyne
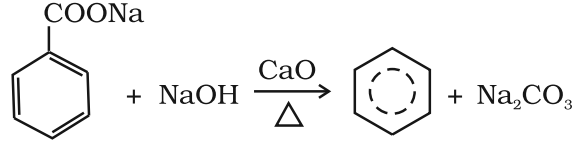
- Decarboxylation of aromatic acids
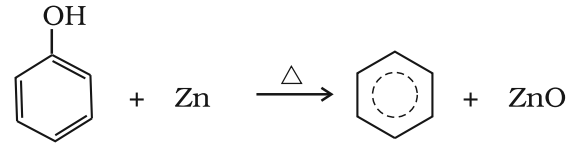
- Reduction of phenol: Phenol is reduced to benzene by passing its vapours over heated zinc dust.
Physical properties
- Aromatic hydrocarbons are non- polar molecules and are usually colourless liquids or solids with a characteristic aroma.
- Aromatic hydrocarbons are immiscible with water but are readily miscible with organic solvents.
- They burn with sooty flame.
Chemical Properties
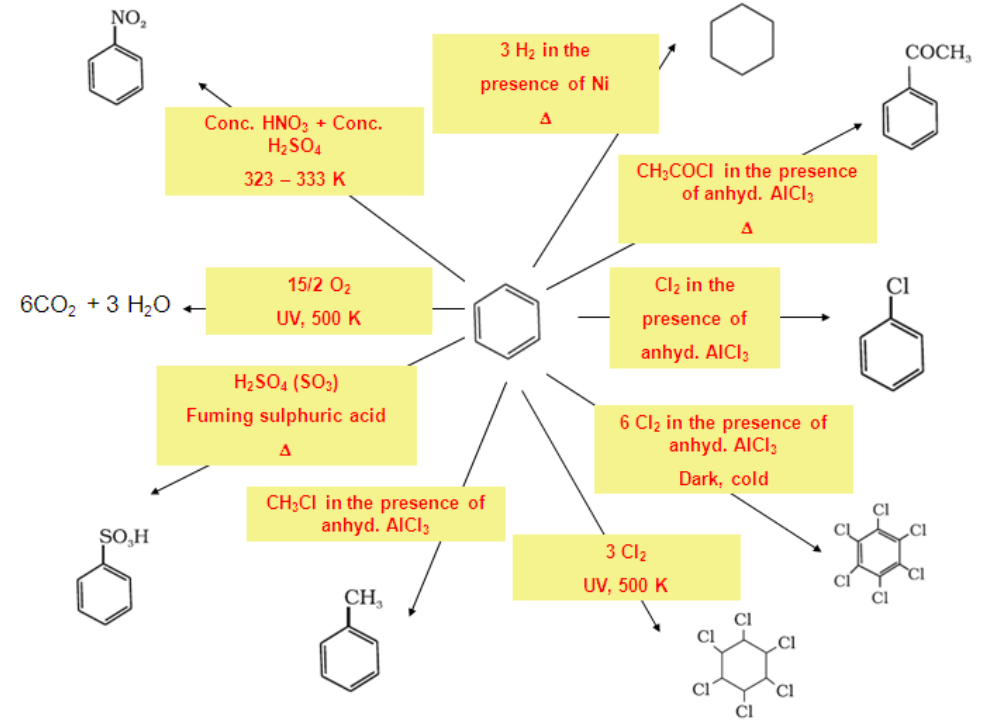
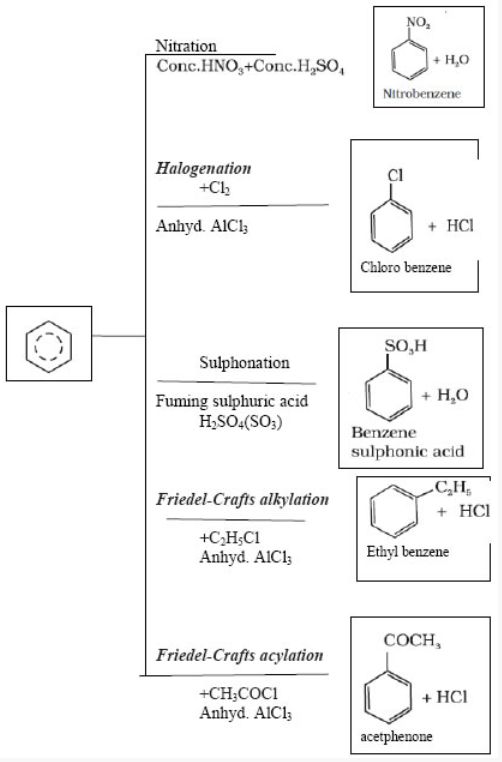
Electrophilic Substitution Reactions:
The following three steps are:
- Generation of the electrophile
- Formation of carbocation intermediate
- Removal of proton from the carbocation intermediate
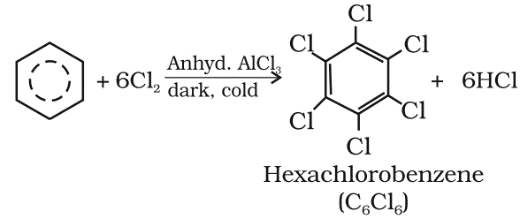
Benzene on treatment with excess of chlorine in the presence of anhydrous AlCl3 can be chlorinated to hexachlorobenzene (C6Cl6).
Mechanism of electrophilic substitution reaction:
All electrophilic substitution reactions follow the same three step mechanism:
Step 1: Generation of the electrophile
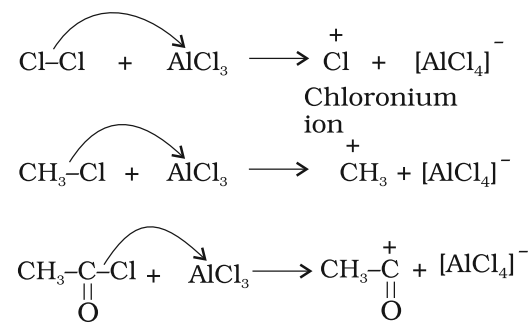
Step 2: Formation of carbocation intermediate
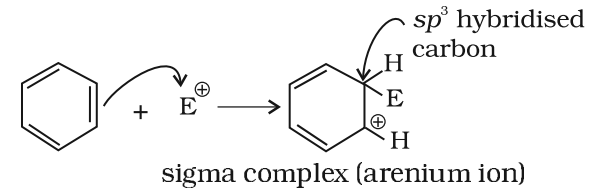
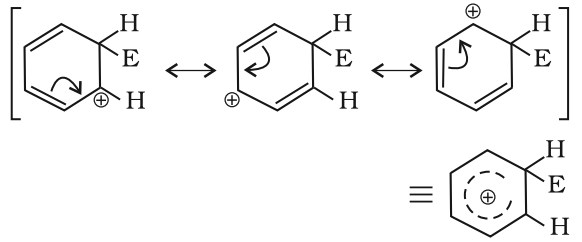
The arenium ion gets stabilized by resonance:
Step 3: Removal of proton from the carbocation intermediate
Activating group:
These group activates the benzene ring for the attack by an electrophile.
Example, —OH; —NH2, —NHR, —NHCOCH3, —OCH3 etc.
Deactivating group:
Due to deactivating group because of strong -I effect, overall electron density on benzene ring decrease. It makes further substitution difficult.
Metadirecting group:
The groups which direct the incoming group to meta position are called meta directing groups. Some examples of meta directing groups are —N02, —CN, —CHO, —COR, —COOH, —COOR, etc.
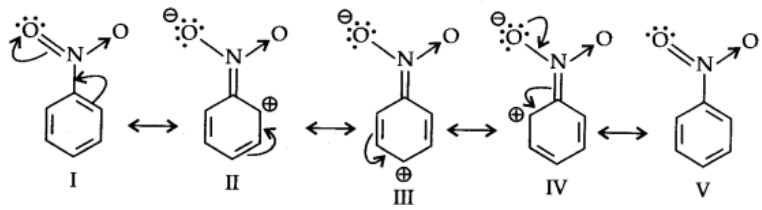
Let us consider the example of nitro group: Since Nitro group due to its strong -I effect reduces the electron density in benzene ring. Nitrobenzene is a resonance hybrid of following structures.
Carcinogenicity and Toxicity
- Benzene and polynuclear hydrocarbons containing more than two benzene rings fused together are toxic and said to possess cancer producing (carcinogenic) property.
- They are actually formed due to incomplete combustion of some organic materials like tobacco, coal and petroleum, etc.
Some of the carcinogenic hydrocarbons are given below: