
H2O2 was discovered by J.L. Thenard in 1818. It is an important compound used in pollution control treatment of domestic and industrial effluents.
Preparation of Hydrogen Peroxide:
From Barium peroxide
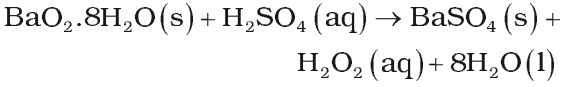
By electrolysis of 50% H2SO4
Electrolysis of a cold 50% solution of H2SO4 at high current density in an electrolytic cell using pe as anode and graphite as cathode.
![]()
At Cathode:
![]()
At Anode:
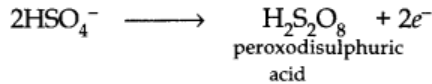
peroxodisulphuric acid formed around anode is withdrawn and then distilled with water under reduced pressure.
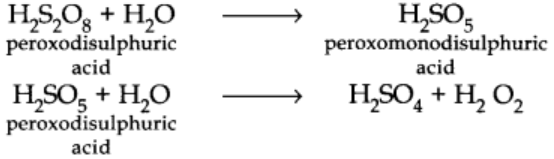
Physical Properties of Hydrogen Peroxide
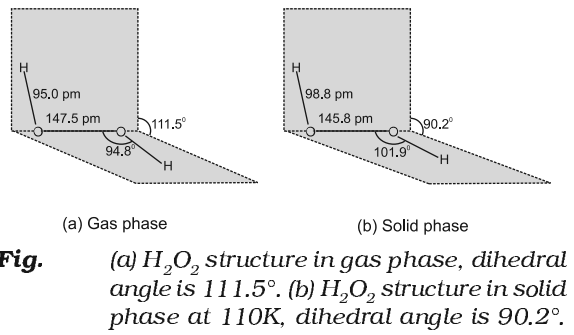
Structure of Hydrogen Peroxide
Hydrogen peroxide has a non-planar structure. The molecular dimensions in the gas phase and solid phase are shown in fig.
Storage of Hydrogen Peroxide (H2O2)
It is stored in the presence of traces of alcohol, acetanilide or sodium pyrophosphate which slow down the rate of decomposition of hydrogen peroxide.
Chemical Properties of H2O2
Acidic nature of H2O2:
It is weakly acidic in nature and pure hydrogen peroxide turns blue litmus red.
H2O2 as Oxidising agent:
It acts as a strong oxidising agent in acidic as well as in basic medium.
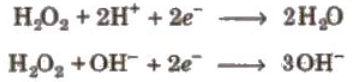
H2O2 as Reducing agent:
- In acidic medium
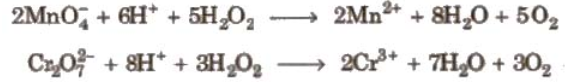
- In basic medium
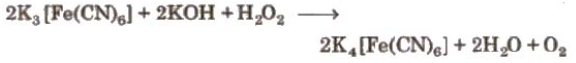
Bleaching properties:
Its bleaching action is due to oxidation by atomic oxygen and permanent.
![]()
dye + [O] dye is oxidized and bleached
Uses of H2O2:
- It is used as a mild disinfectant. It is marketed as perhydrol (an Antiseptic).
- It is used in the manufacture of high quality detergents.
- It is used in the synthesis of hydroquinone tartaric acid and certain food products and pharmaceuticals.
- It is used as bleaching agent for textiles, paper pulp etc.
- It is used for pollution control treatment of domestic and industrial effluents.
- 93% H202 is used as an oxidant for rocket fuel.

