
At a constant temperature, the rate of a chemical reaction is directly proportional to the product of the molar concentrations of the reactants each raised to a power equal to the corresponding stoichiometric coefficients as represented by the balanced chemical equation. Let us consider the reaction,
aA + bB +… ⟶ products
Rate of reaction α [A]a[B]b……
By the law, rate of reaction = k[A]a[B]b…
Here a and b are stoichiometric coefficients. K is the rate constant.
Let us consider a general reversible reaction
A + B ⇌ C + D
Applying Law of Mass Action,
Rate of the forward reaction α[A][B] = Kf[A][B]
When Kf is a constant of proportionality and is called velocity constant for the forward reaction.
Rate of the backward reaction α[C][D] = Kb[C][D]
At equilibrium,
Rate of the forward reaction = Rate of the backward reaction
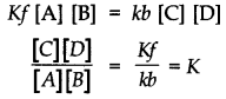
At constant temperatures K is also constant and is called Equilibrium constant.
Now let us consider a more general reversible reaction in a state of equilibrium. By applying Law of mass action.
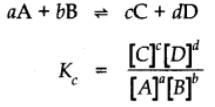
Equilibrium Constant for the reaction

Relationship between Equilibrium constant K, reaction Quotient Q and Gibbs energy G.
A mathematical expression of thermodynamic view of equilibrium can be described by time equation.
![]()
where G⊖ is standard Gibbs energy.
At equilibrium when ∆G = 0
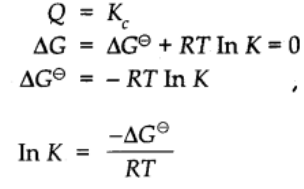
Taking antilog on both sides
![]()

