
When rate of formation of a product in a process is in competition with rate of formation of reactants, the state is then named as “Equilibrium state”.
Equilibrium may be classified as:
Physical Equilibrium
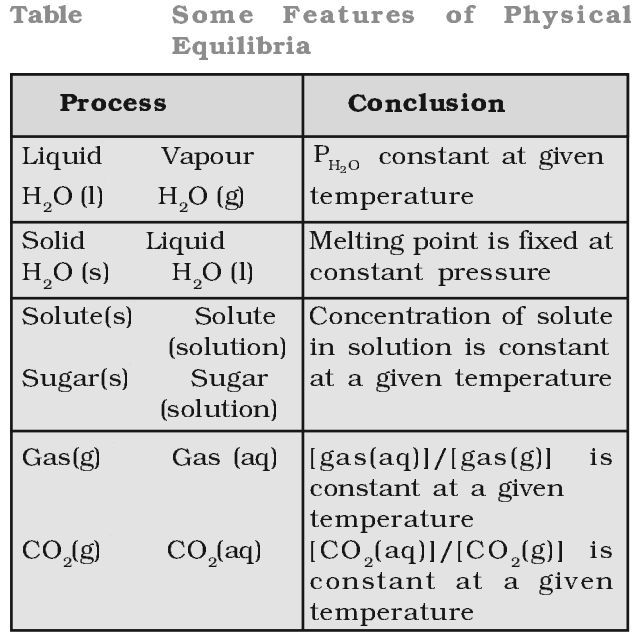
Equilibrium set up in physical processes like evaporation of water, melting of solids, dissolution of solutes, etc., is called physical equilibrium, e.g., Ice ⇔ Water
At equilibrium,
Rate of melting of ice = Rate of freezing of water
Chemical Equilibrium
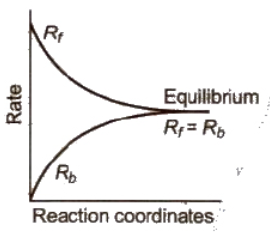
If a reversible reaction is carried out in a closed vessel, a stage is attained where the speed of the forward reaction equals the speed of the backward reaction. It corresponds to chemical equilibrium.
At equilibrium,
Rate of forward reaction = Rate of backward reaction
Characteristics of Chemical Equilibrium
- At equilibrium, the concentration of each of the reactants and the products becomes constant
- At equilibrium, the rate of forward reaction becomes equal to the rate of backward reaction and hence the equilibrium is dynamic in nature.
- A chemical equilibrium can be established only if none of the products is allowed to escape out or separate out as a solid.
- Chemical equilibrium can be attained from either direction, i.e., from the direction of the reactants as well as from the direction of the products.

