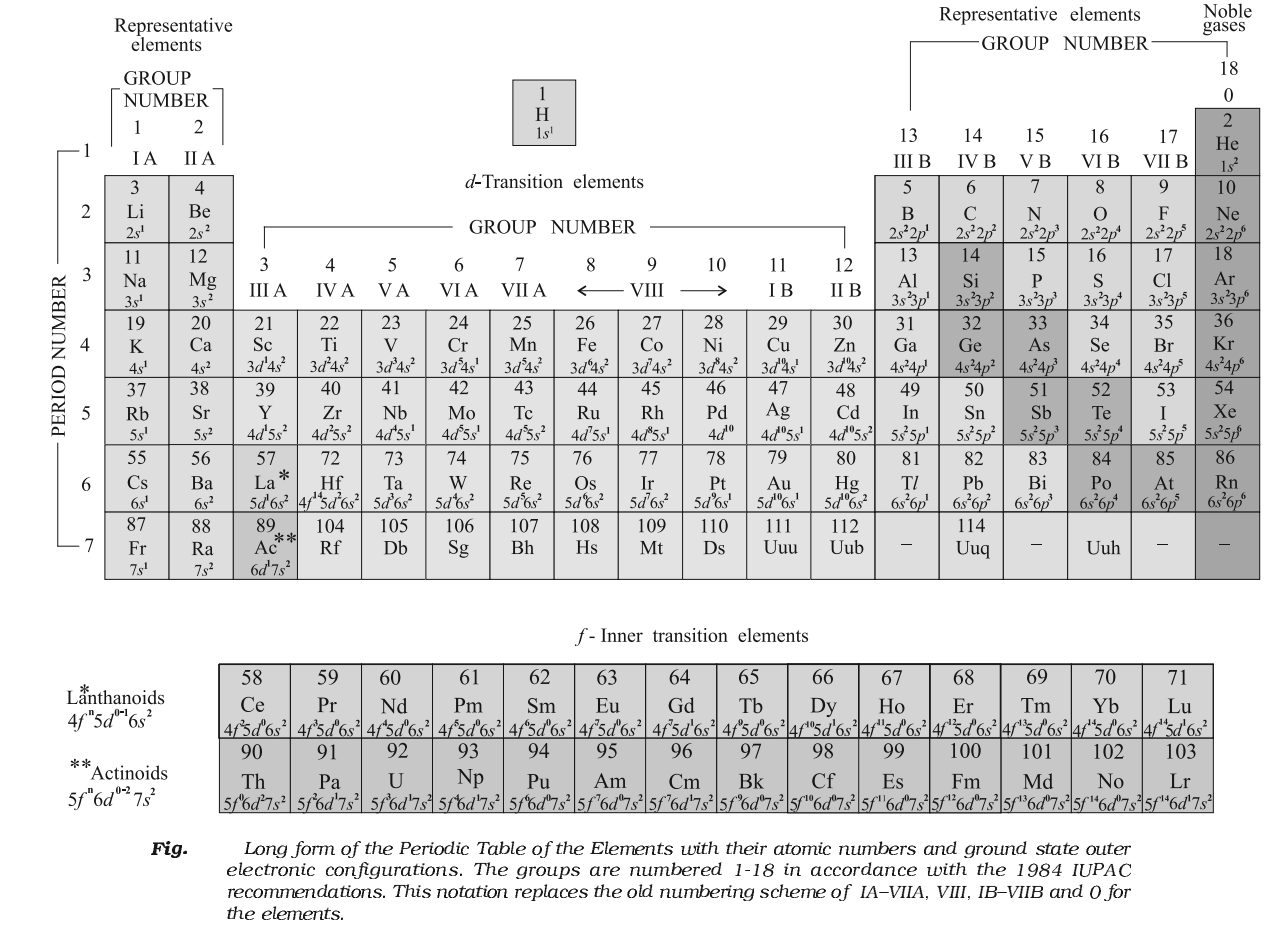
Modern Periodic table (1913)
- Mostly modified Mendeleev’s periodic law. He stated “Physical and chemical properties of elements are the periodic function of their atomic numbers.”
- It is known as modern periodic law and considered as the basis of Modern Periodic Table.
- In this table, the elements have been arranged in increasing atomic number,
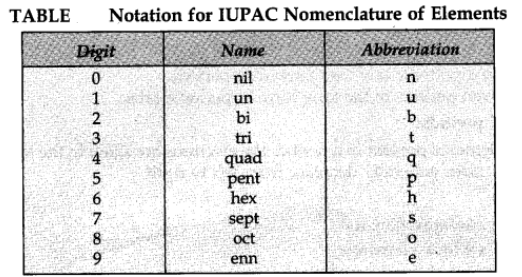
Nomenclature of elements with Atomic number more than 100

Structural Features of the Periodic Table
Group:
The long form of periodic table also consists of the vertical rows called groups. There are in all 18 groups in the periodic table. Unlike the Mendeleev periodic table, each group is an independent group.
Characteristics of groups:
- All the elements present in a group have the same general electronic configuration of the atoms.
- The elements in a group are separated by definite gaps of atomic numbers (2,8,8,18,18,32).
- The atomic sizes of the elements in the group increase down the group due to increase the number of shells.
- The physical properties of the elements such as m.p., b.p. density, solubility etc., follow a systematic pattern.
- The elements in each group have generally similar chemical properties.
Periods:
Horizontal rows in a periodic table are known as periods. There are in all seven periods in the long form of periodic table.
Characteristics of periods:
- In all the elements present in a period, the electrons are filled in the same valence shell.
- The atomic sizes generally decrease from left to right.
s-Block Elements
General electronic configuration: ns1-2
Characteristics of s-block elements:
- All the elements are soft metals.
- They have low melting and boiling points.
- Most of them impact colors to the flame.
- They are highly reactive.
- They generally form ionic compounds.
- They are good conductors of heat and electricity.
p-Block Elements
General electronic configuration: ns2np1-6
Characteristics of p-block elements:
- The compounds of these elements are mostly covalent in nature.
- They show variable oxidation states.
- In moving from left to right in a period, the non-metallic character of the elements increases.
- The reactivity of elements in a group generally decreases downwards.
- At the end of each period is a noble gas element with a closed valence shell ns2np6 configuration.
- Metallic character increases as we go down the group.
d-Block Elements
- General electronic configuration: (n-1)d1-10ns0-2
- The d-block elements are known as transition elements because they have incompletely filled d-orbitals in their ground state or in any of the oxidation states.
Characteristics of d-block elements:
- They are all metals with high melting and boiling points.
- The compounds of the elements are generally paramagnetic in nature.
- The mostly form colored ions, exhibit variable valence (oxidation states).
- They are of tenly used as catalysts.
f-Block Elements
- General electronic configuration: (n-2)f1-14(n-1)d0-1ns2
- They are known as inner transition elements because in the transition elements of d-block, the electrons are filled in (n-1) d sub-shell while in the inner transition elements of f-block the filling of electrons take place in (n-2) f sub-shell, which happens to be one inner subshell.
Characteristics of f-block elements:
- The two rows of elements at the bottom of the Periodic Table, called the lanthanoids Ce (Z=58) – Lu (Z=71) and Actinoids Th (Z=90) – Lr (Z = 103).
- These two series of elements are called Inner Transition Elements (f-Block Elements).
- They are all metals. Within each series, the properties of the elements are quite similar.
- Most of the elements pf the actinoid series are radio-active in nature.
Metals:
- Metals comprise more than 78% of all known elements and appear on the left side of the Periodic Table.
- Metals are solids at room temperature.
- Metal usually have high melting and boiling points.
- They are good conductors of heat and electricity.
- They are malleable and ductile.
Non-metals:
- Non-metals are located at the top right-hand side of the Periodic Table.
- Non-metals are usually solids or gases at low temperature with low melting and boiling point.
- They are poor conductors of heat and electricity.
- The non-metallic character of heat and electricity.
- The non-metallic character increases as one goes from left to right across the Periodic table.
- Most non-metallic solids are brittle and are neither malleable nor ductile.
Metalloids:
The elements (e.g., silicon, germanium, arsenic, antimony and tellurium) show the characteristic of both metals and non-metals. These elements are also called semimetal.
Noble Gases:
- These are the elements present in group 18.
- Each period ends with a noble gas element.
- All the members are of gaseous nature and because of the presence of all the occupied filled orbitals, they have very little tendency to take part in chemical combination.
- These are also called inert gases.
Representative Elements:
- The elements of group 1 (alkali metals), group 2 (alkaline earth metals) and group 13 and 17 constitute the representative elements. They are elements of s-block and p-block.
Transition Elements:
- The transition element includes, all the d-block elements and they are present in the center of the periodic table between s and p-block elements.
Inner Transition Elements:
- Lanthanoids (the fourteen elements after Lanthanum) and actinides (the fourteen elements after actinium) are called inner transition elements. They are also called f-block elements.
- The elements after uranium are also called transuranic elements.