
Carbon Monoxide, CO
Preparation of Carbon Monoxide:
It is prepared by direct oxidation of C in limited supply of oxygen.
![]()
On small scale it is prepared by dehydration of formic acid with Con. H2SO4 at 373K.
![]()
Properties:
- It is colourless, odourless and almost water insoluble gas.
- It a powerful reducing agent. CO is used in the extraction of many metals from their oxide ores.

Carbon Dioxide CO2
Preparation of Carbon Dioxide:
It is prepared by complete combustion of carbon and carbon containing fuels in excess of air.
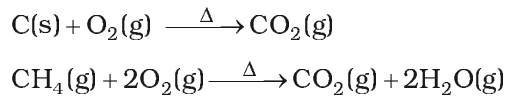
Properties:
- It is a colourless and odourless gas.
- It is slightly soluble in water. When C02 dissolves in water only some of the molecules react with water to form carbonic acid.
- It is not poisonous like CO.
- But increase in combustion of fossil fuels and decomposition of limestone for cement manufacture increase of C02 in the atomosphere is one of the main reasons of green-house effect.
- Photosynthesis
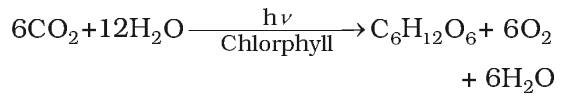
Silicon Dioxide, SiO2
Silicon dioxide, commonly known as silica, occurs in various crystallographic forms.
For example, Quartz, Cristobalite and thermite are some of the crystalline forms of silica.
Structure of Silicon Dioxide:
Silicon dioxide is a covalent three-dimensional network solid. Each silicon atom is covalently bonded in a tetrahedral manner to four oxygen atoms. Each oxygen atom in turn covalently bonded to another silicon atoms as shown below:
![]()
Properties of Silicon Dioxide:
- In normal form silica is very less reactive.
- At elevated temperature it does not reacts with halogens, dihydrogen and most of the acids and metals. But it reacts with HF and NaOH.
![]()
Uses of Silicon Dioxide:
- Quartz is extensively used as a piezoelectric material.
- Silica gel is used as adsorbent in chromatography.
- An amorphous form of silica, kieselghur is used in filtration plants.
Silicones
Preparation of Silicones:
The linear, cyclic or cross-linked polymeric compounds containing (R2SIO) as a repeating unit, are known as silicones. They are manufactured from alkyl substituted chlorosilanes.
![]()
Properties of Silicones:
- It is chemically inert, water repellent, heat resistant.
- It is good electrical insulators.
Uses of Silicones:
- It is used as lubricants (Vaseline), insulators etc.
Silicates
- A large number of silicates minerals exist in nature. Some of the examples are feldspar, zeolites, mica and asbestos. The basic structural unit of silicates is SiO-44.
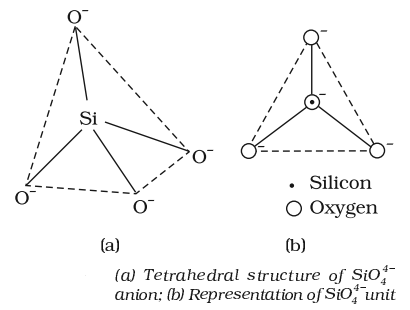
- Two important man-made silicates are glass and cement.
Zeolites
- Aluminosilicate are formed when few Si atoms are replaced by Al atoms in three-dimensional network of silicon dioxide.
- Zeolites are widely used as a catalyst in petrochemical industries for cracking of hydrocarbons and isomerisation,
- e.g., ZSM-5 (A type of zeolite) used to convert alcohols directly into gasoline.
- Zeolites are complex aluminium silicates.
- Hydrates zeolites are used as ion exchangers in softening of “hard” water.

