
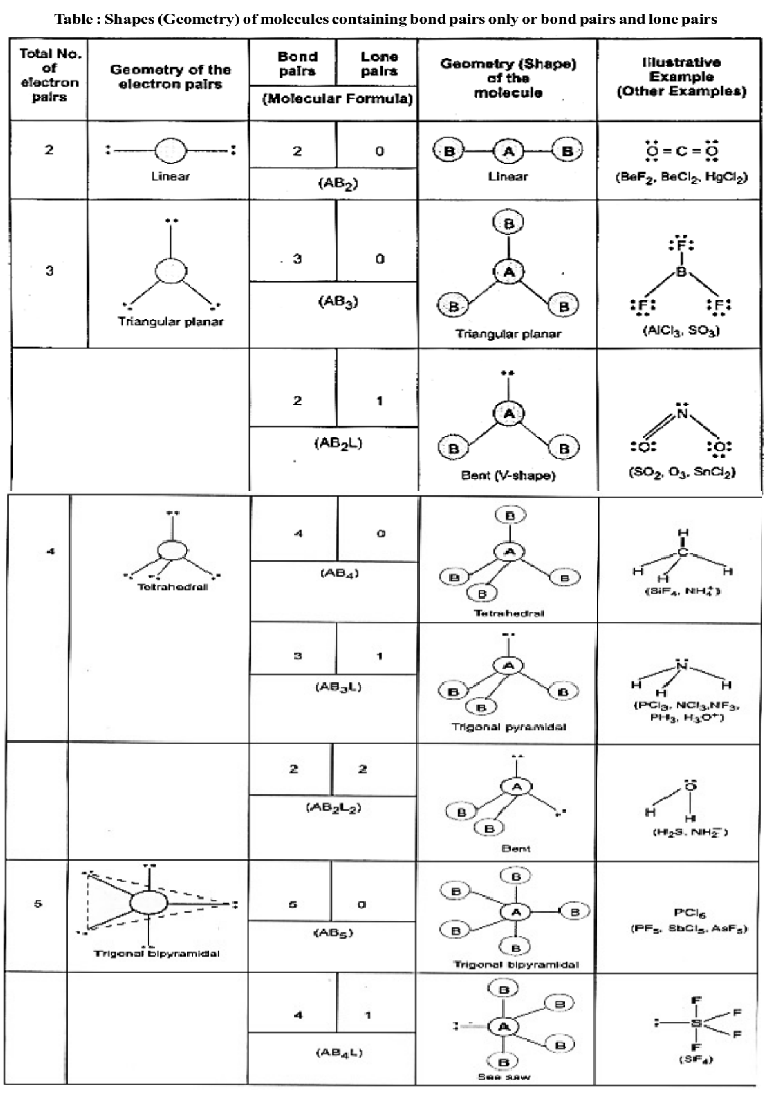
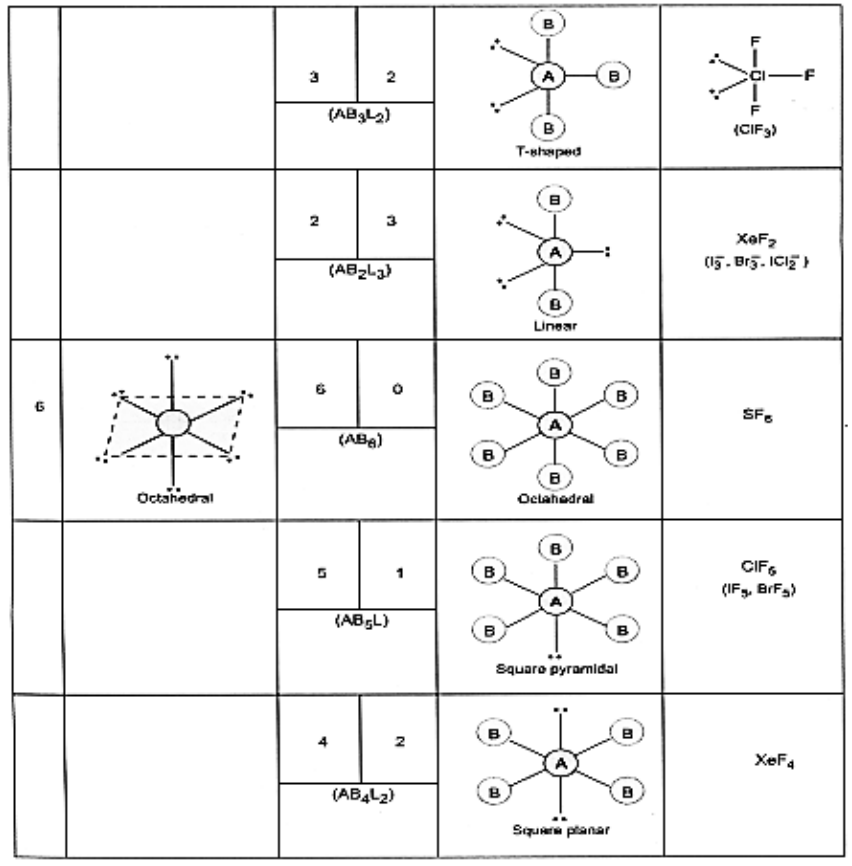
Valence Shell Electron Pair Repulsion Theory
According to this theory,
- The geometry of a molecule or ion depends on the number of electron pairs in the valence shell of its central atom.
- To attain minimum repulsive state electron pairs, try to stay as far away as possible.
- If the central atom is surrounded by only bonded electron pairs of similar atoms, the repulsive interactions are similar and the molecular geometry is regular.
- If the central atom is surrounded by one bonded electron pairs of dissimilar atoms, the repulsive interactions are not equivalent and hence, the geometry of molecule will not be regular.
- If the central atom is surrounded by both bonded pairs (bp) as well as lone pairs (lp) of electrons. Repulsive interactions are not equivalent and hence, geometry of the molecule will be irregular.
The repulsive interactions decrease in the order
lp – lp > lp – bp > bp – bp
Valence Bond Theory
Valence bond theory was introduced by Heitler and London (1927) and developed by Pauling and others. It is based on the concept of atomic orbitals and the electronic configuration of the atoms.
Let us consider the formation of hydrogen molecule based on valence-bond theory.
Let two hydrogen atoms A and B having their nuclei NA and NB and electrons present in them are eA and eB.
As these two atoms come closer new attractive and repulsive forces begin to operate.
- The nucleus of one atom is attracted towards its own electron and the electron of the other and vice-versa.
- Repulsive forces arise between the electrons of two atoms and nuclei of two atoms. Attractive forces tend to bring the two atoms closer whereas repulsive forces tend to push them apart.

