- General formula: CnH2n+2
- Each carbon atom of alkane is sp3 hybridized and its shape is tetrahedral.
- The simplest member of alkane series is methane (CH4), ethane (C4H6), propane (C3H8), etc.
- These hydrocarbons are inert under normal conditions [i.e., do not react with acids, bases and other reagents).
- Hence, they were earlier known 88 paraffins (Latin: parum-little; affins-affinity)
- Alkanes exhibit chain isomensm, position isomerism and conformational isomerism.
Structure of Alkanes
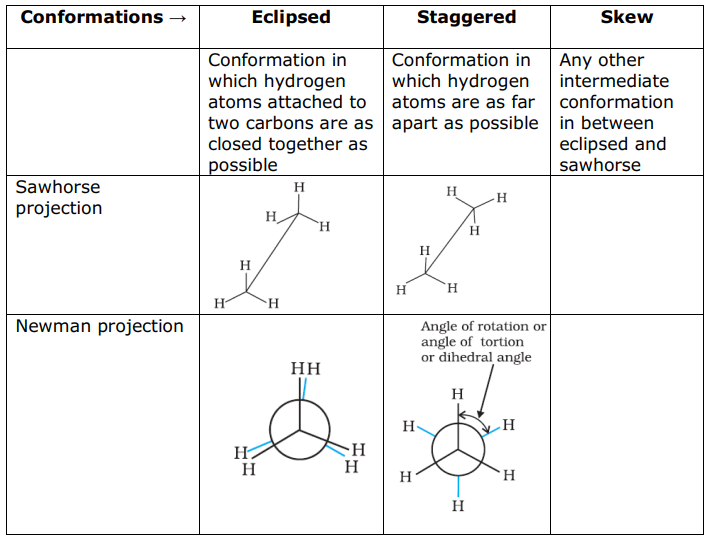
In methane carbon forms single bonds with four hydrogen atoms. All H—C—H bond angles are of 109.5°. Methane has a tetrahedral structure. C—C and C—H bonds are formed by head-on overlapping of sp3 hybrid orbitals of carbon and Is orbitals of hydrogen atoms.
Nomenclature of Alkanes
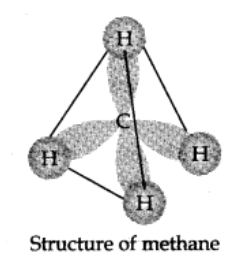
Use the following step-by-step procedure to write the IUPAC names from the structural formulas. Consider the following structural formula:
Step 1: Identify the longest chain:
In the given example, longest chain has seven carbons. The seven-carbon chain is heptane.

Step 2: Number the chain:
The chain is numbered from left to right. This gives lowest numbers to the attached alkyl group.
Step 3: Identify the alkyl group:
There are two methyl groups at C-2 and C-3, there is one ethyl group of C-4.
Step 4: Write the IUPAC name:
In this case the IUPAC name is 4-Ethyl-2,3-dimethyl heptane. Always keep in mind
- Numbers are separated from each other by commas.
- Numbers are separated from names by hyphens
- Prefixes di, tri is not considered in alphabetizing substituent names.
Preparation of Alkanes:
From unsaturated hydrocarbon
By adding Dihydrogen gas adds to alkenes and alkynes in the presence of finely divided catalysts like platinum, palladium or nickel to form alkanes. This process is called hydrogenation.
For example:
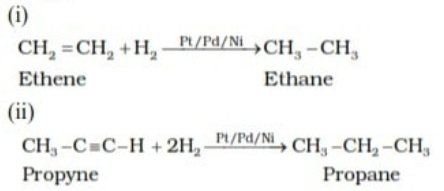
From alkyl halides
On reduction with zinc and dilute hydrochloric acid
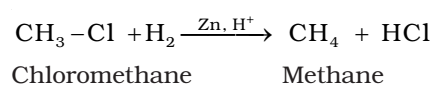
Wurtz Reaction
Used for the preparation of higher alkanes

From carboxylic acids
By elimination carbon dioxide from carboxylic acid
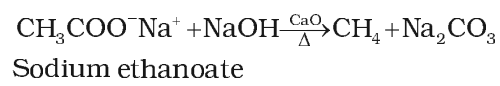
Kolbe’s electrolytic method
On electrolysis of aqueous solution of sodium or potassium salt of a carboxylic acid gives alkane containing even number of carbon atoms.
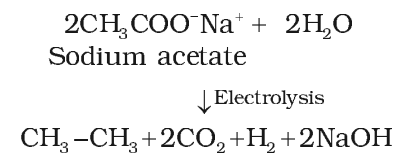
Physical Properties of Alkanes:
- They are hydrophobic and dissolve in nonpolar or weakly polar solvents.
- They are less dense than water.
- The boiling point of alkane increases by 20-30 degree Celsius with each additional methylene group.
- For isomeric alkanes, the boiling point decreases with branching due to an overall increase in surface area.
Chemical Properties of Alkanes

Alkanes are extremely stable and inert substance due to presence of non-polar C – C and C – H bonds. Alkanes are saturated compounds with strong sigma bonds which doesn’t break under ordinary conditions. Alkanes react at high temperature by free radical mechanism.

Substitution Reactions of Alkanes
Substitution Reactions are those in which an atom or group of atoms in a molecule is replaced by some other atom or group of atoms.
Halogenation (free radical substitution):
This involves the replacement of one or more atoms of alkanes by the corresponding number of halogen atoms. For example:
Chlorination
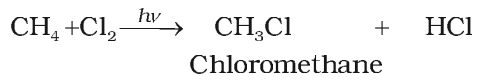
Bromination of alkanes proceeds in the same way but not so easily.
Iodination
![]()
Order of reactivity of halogens is
![]()
Order of reactivity of hydrogen of alkane is
![]()
Mechanism:
Mechanism of halogenation of alkanes is free radical in nature, i.e., the attacking reagent is a halogen free radical (X). It is a chain reaction.
Chain initiation step:
The reaction is initiated by homolysis of chlorine molecule in the presence of light or heat. The Cl–Cl bond is weaker than the C–C and C–H bond and hence, is easiest to break.

Chain Propagation step:
Chlorine free radical attacks the methane molecule and takes the reaction in the forward direction by breaking the C-H bond to generate methyl free radical with the formation of H-Cl.
![]()
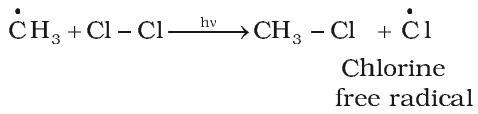
Chain Termination step:
The reaction stops after some time due to consumption of reactants and / or due to the following side reactions:
The possible chain terminating steps are:
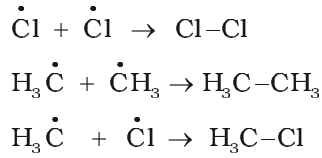
Nitration of Alkanes:
- The reaction takes places by free radicals mechanism at high temp (450oC).
- At high temp C-C bond is also broken so that mixture of nitroalkanes is obtained.
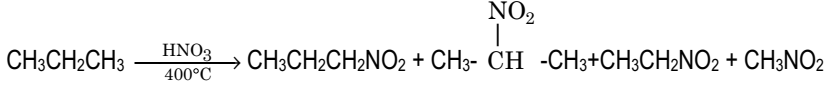
- Sulphonation: Replacement of hydrogen atom of alkane by -SO3H group.

Oxidation of Alkanes
Combustion:
On heating in presence of air, they are completely oxidized to carbon dioxide with large amount of heat.

Controlled Oxidation:
Alkanes on heating with a regulated supply of dioxygen or air at high pressure and in the presence of suitable catalysts give a variety of oxidation products.
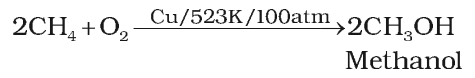
Action of steam
Methane reacts with steam at 1273 K in the presence of nickel catalyst to form carbon monoxide and dihydrogen. This method is used for industrial preparation of dihydrogen gas.
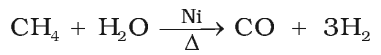
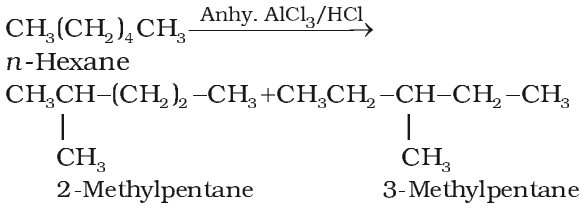
Isomerisation
Alkanes isomerise to branched chain alkanes when heated with anhydrous aluminium chloride (AlCl3) and hydrogen chloride gas at 573 K under a pressure of about 30-35 atmosphere.
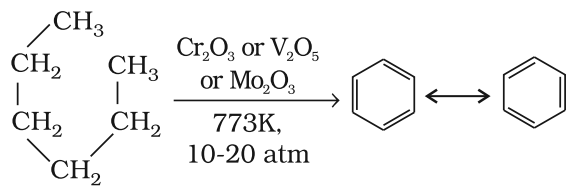
Aromatization
n-Alkanes having six or more carbon atoms on heating to 773K at 10-20 atmospheric pressure in the presence of oxides of vanadium, molybdenum or chromium supported over alumina get dehydrogenated and cyclised to benzene and its homologues. This reaction is known as aromatization or reforming.
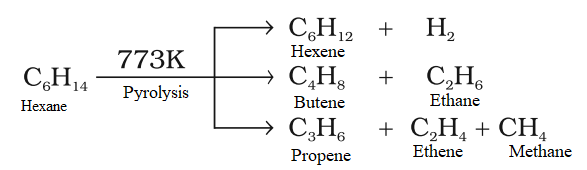
Pyrolysis or Thermal decomposition
when higher alkanes are heated at high temp (about 700-800k) in the presence of alumina or silica catalysts, the alkanes break down to lower alkanes and alkenes.
Conformations
The spatial arrangements of atoms which can be converted into one another by rotation around a C-C single bond are called conformations or conformers or rotamers.
Conformations of ethane: