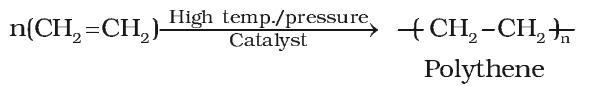- Alkenes are characterized by the presence of a double bond between two carbon atoms.
- Alkenes have the general formula CnH2n.
- C-C bond hybridization 1.34 Ao
- Sp2 hybridization
- When we treated Alkene with chlorine, oily products are obtained. So, Alkenes are also known as Olefins. (Greek olefiant meaning oil forming).
- Show chain, positional and geometrical isomerism.
Structure of double bond (ethene)
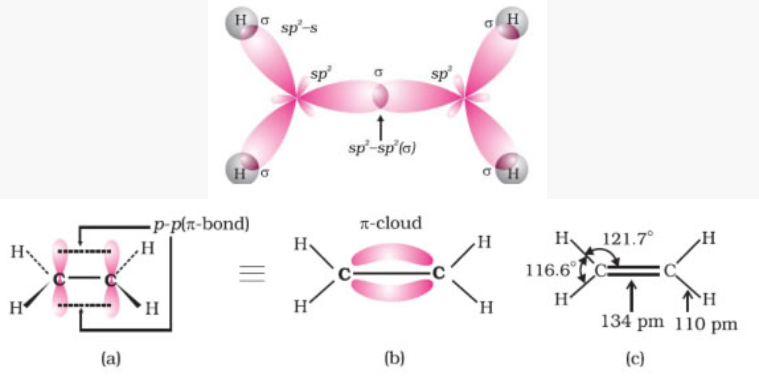
Fig. Orbital picture of ethene showing formation of (a) π-bond, (b) π-cloud and (c) bond angles
and bond length
Methods of preparation
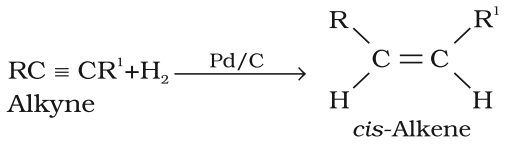
Preparation of Alkene from Alkynes
- On partial reduction of alkyne with calculated amount of dihydrogen in the presence of Lindlar’s catalyst give alkynes.
Preparation of Alkene from Haloalkanes (dehydrohalogenation)
- On heating with alcoholic potash one molecule of halogen acid is eliminated to form alkenes.
- The above process is called dehydrohalogenation
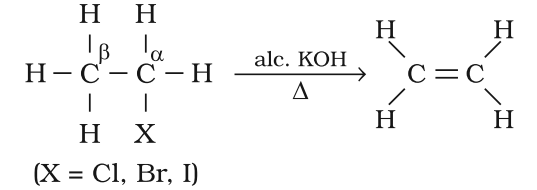
- For halogens the rate of reaction is:
Iodine > Bromine > Chlorine
- For alkyl groups the rate of reaction is:
tert > secondary > primary
Preparation of Alkene from Dihaloalkanes (dehalogenation)
- Dihalides in which two halogen atoms are attached to two adjacent carbon atoms are known as vicinal dihalides.
- Vicinal dihalides goes dehalogenation for the formation of alkenes.
![]()
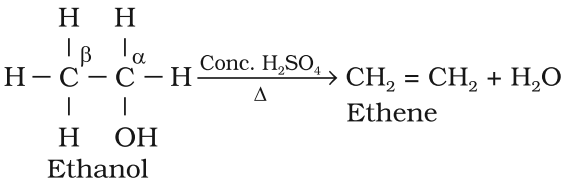
Preparation of Alkene from Alcohols (dehydration)
- On heating alcohols with concentrated sulphuric acid.
- Above process is known as acidic dehydration of alcohols.
Nomenclature of Alkenes
In IUPAC system
- The name of the hydrocarbon is based on the parent alkene having the longest carbon chain of which double bond is apart.
- The suffix ‘ene’ replaces ‘ane’ of alkanes.
Isomerism
Alkenes show both structural isomerism and geometrical isomerism
Structural isomerism
- Ehtene and propene have no structural isomers, but there are three structure of butenes.
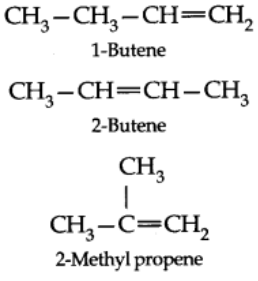
- Of these, two are straight chain structure with the difference being in the position of double bond in the molecules.
- These are position isomers and third structure is a branched-chain isomer.
Geometrical Isomerism
- It is known that a carbon-carbon double bond is made up of one σ bond and one π-bond.
- The π-bond presents free rotation about the double bond.
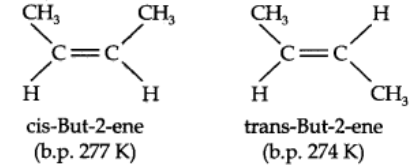
This presentation of rotation about the carbon-carbon double bond gives rise to the phenomenon of geometrical isomerism. - An alkene having a formula RCH=CHR can have two stereoisomers, depending upon whether the two alkyl groups are on the same or opposite sides of the double bond.
- If they are on the same side, then it is called cis-isomer. If they are on opposite sides, then it is called trans-isomer.
- Due to different arrangement of atoms or groups in space, these isomers differ in their properties like melting point, boiling point, dipole moment, solubility, etc.
Physical properties of alkenes
- Physical state: The first three members are gases, the next fourteen are liquids and the higher ones are solids.
- Boiling Points: The boiling points of alkenes increases with the increase in molecular mass.
- Solubility: Alkenes are soluble in non-polar solvents and are insoluble in polar solvents.
Chemical reaction of alkenes
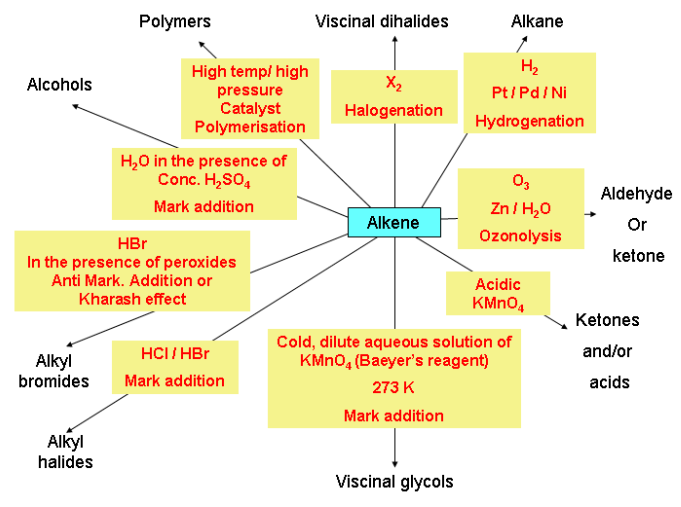
Alkenes are reactive due to the presence of double bonds. Due to presence of π bonds alkenes give electrophilic addition reaction. Alkenes also give free radical addition reaction.
Addition reaction:
Alkene show electrophilic addition reaction.
Addition of hydrogen (Catalytic hydrogenation)
![]()
Addition of halogens (Cl2 or Br2)
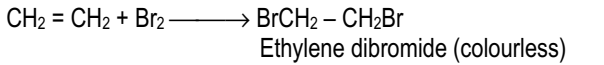
Addition of bromine is used as a test for detecting the presence of a carbon-carbon double bond or triple bond.
Addition of hydrogen halides
![]()
The order of reactivity among hydrogen halides is
![]()
In case of unsymmetrical alkenes addition occurs according to Markonikov’s rule. This reaction takes place through an ionic mechanism. Electrophilic addition to a carbon–carbon double bond involves the formation of an intermediate, i.e. more stable carbocation.
Deviation from Markownikov’s rule:
Negative paet of the addendum (adding molecule) gets attached to that carbon atom which possesses lesser number of hydrogen atoms. e.g.,
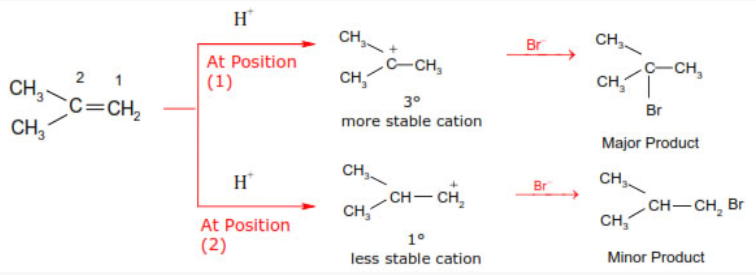
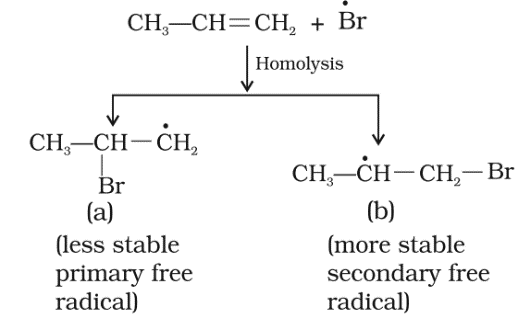
Peroxide effect or Kharasch (Anti Markownikoff’s addition):
In 1933 Kharasch and Mayo observed that when HBr is added to an unsymmetrical double bond in the presence of organic peroxide, the reaction take places opposite to the Markovnikov rule.

Addition of Sulphuric acid:
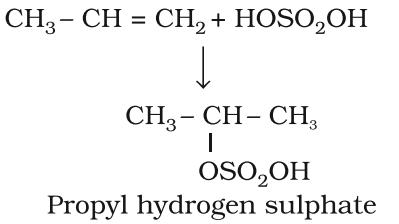
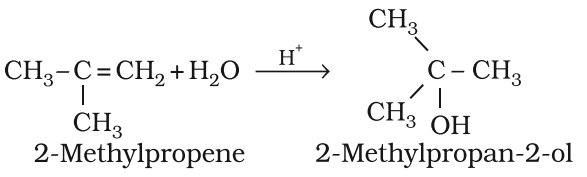
Addition of water (Hydration):
Acid catalyzed addition of water.
Oxidation of Alkenes
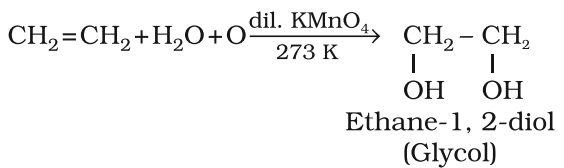
Alkenes decolourise cold dilute aqueous solution of potassium permanganate (Baeyer’s reagent). It is used as a test for unsaturation.
Acidic KMnO4 or acidic K2Cr2O7 oxidise alkenes to ketones and/or acids depending upon the nature of alkene and the experimental conditions.
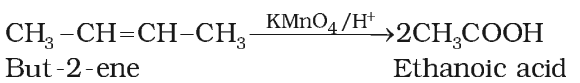
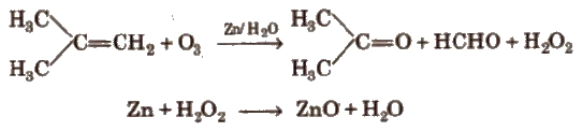
Ozonolysis of Alkenes
Alkenes involves the addition of ozone molecule to alkene to form ozonide, and then cleavage of the ozonide by Zn-H2O to smaller molecules
Polymerisation
- Polythene is obtained by the combination of large number of ethene molecules at high temperature, high pressure and in the presence of a catalyst. The large molecules thus obtained are called polymers. This reaction is known as polymerisation.
- The simple compounds from which polymers are made are called monomers.